SWAB, cotton tip, wooden stick, 15 cm, ste, s.u.
Valid Article
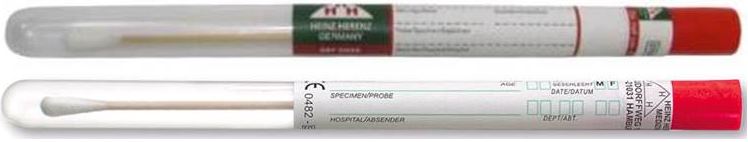
SWAB, cotton tip, wooden stick, 15 cm, sterile
Definition
Sterile dry swab used to take samples, contained in labelled a cylindrical tube.
Specifications
Technical specifications
- Plastic tube (PP) with label Ø 12 x 155 mm
- Wooden stick with cotton bud, ± 16 x 150 mm, fixed to the cap of the tube
- Sterile, for single use
Packaging & Labelling
Unit sterile packaging. Box of 100.
Instructions for use
Please consult the “Updated laboratory procedures, 2022” or “Bacteriology laboratory procedures and resources“ available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Home.aspx
For offline access, contact your laboratory advisor.
Storage
- Store between 2 and 25°C
- Shelf life: 36 months
MSF requirements
Swabs are sterile and ready-for-use systems intended for collected clinical samples to be sent for cultural examination.
Swabs for general purpose: suitable for throat, vagina, skin.
Caution: Swabs with wooden sticks are not suitable for PCR analysis.