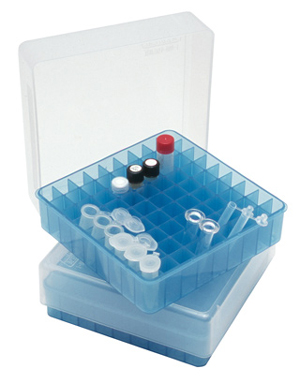
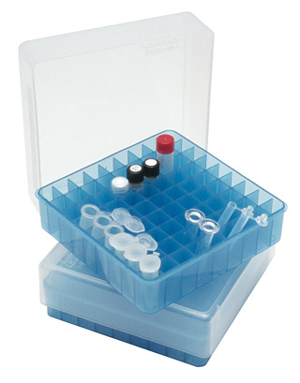
STORAGE BOX, PP, 9x9 microtubes 1-2ml, autoclavable
STD
ELABTUMB81PP
Valid Article
Account code:
60200
HS Code:
901890
Last Updated on:
29/06/2025, 22:36:20
Former
Code(s):
ELAETUMI1B- ELAEZBE0887 ELABTUMI887 ELABTUMIR81
W050301020280 - Samples analyses, plastic microtubes - accessories
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
STORAGE BOX, PP, 9x9 microtubes 1-2ml, autoclavable
Definition
Polypropylene box to store microtubes. Can be reused. Resistant to freezing and autoclaving.
Specifications
Material
Polypropylene
Technical specifications
- Capacity: 81 cryotubes from 1.2 to 2 ml
- Range temperature tolerance: from -196°C to 121°C
- Autoclavable
- L x W x H: 130 x 130 x 47 mm
- Reusable
- Available in different colors
Packaging & Labelling
Order unit: 1 box
Usual packing: 4 or 5 boxes/case
MSF requirements
Autoclavable box to store cryotubes containing antibiotics, reagents, or biological liquid suspension.
Some restricted information has been hidden. Sign in
to see this information




![[ELABTUMC20EN] CRYOTUBE, 2.0 ml, round, ext. thread, non-sterile](/web/image/product.template/571443/image_256/%5BELABTUMC20EN%5D%20CRYOTUBE%2C%202.0%20ml%2C%20round%2C%20ext.%20thread%2C%20non-sterile?unique=01560e3)
![[ELABTUMS20AS] MICROTUBE, 2.0ml, skirted, assembl. attach. screw cap, ster.](/web/image/product.template/569078/image_256/%5BELABTUMS20AS%5D%20MICROTUBE%2C%202.0ml%2C%20skirted%2C%20assembl.%20attach.%20screw%20cap%2C%20ster.?unique=de865de)