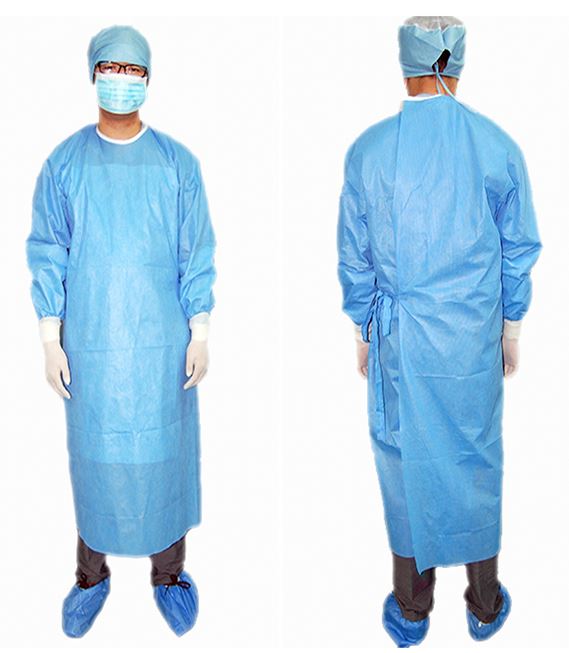
SURGICAL GOWN, non-woven, high performance, sterile, XXL
Valid Article
SURGICAL GOWN, HP, non-woven
Definition
A single use garment made of synthetic materials intended to be worn over a scrub suit to cover the arms, trunk, and upper legs, during a surgical procedure; it may be fluid resistant or impervious to fluids. Also known as an operating room (OR) gown, it is used during surgical procedures to help protect both the patient and operating room personnel from the transfer of microorganisms, body fluids, and particulate material.
Synonym
surgical smock
Specifications
critical product area: product area with a greater probability to be involved in the transfer of infective agents to or from the wound, e.g. front and sleeves of surgical gowns
Quality standards
Quality Standards Comment
EN 13795 test results (gown high performance)
- EN 29073-3: Tensile strength - dry and wet: ≥ 20 Newton (amount in force applied to break the strip)
- EN 13938-1: Burst strength - dry and wet: ≥ 40 kPa strength at burst
- EN 20811: Resistance to fluid penetration: critical area / less critical area: ≥ 100/10 cm of water pressure on the fabric sample
- EN ISO 11737: Cleanliness – microbial: ≤ 300 Number of colonies formed / dm²
- ISO 9073-10: Cleanliness – particulate matter and Linting: Number of particles 3-25µm as an index for PM expressed as Log10: critical area / less critical area: ≤ 3.5 / ≤ 4.0
- ISO 22612: Resistance to microbial penetration – dry: ≤ 300 CFU (number of colonies formed after incubation)
- ISO 22610: Resistance to microbial penetration – wet: for the critical area: ≥ 6.0 Number of colonies formed after incubation, expressed in Barrier Index
Technical specifications
- Non-woven, multilayer (SMS or SMMS)
- Impervious supplementary protection layer (polyethylene film, nonwoven, etc.) in the critical zones (chest area and forearms) = reinforced
- For medium to high fluid level interventions
- Wraparound
- Long sleeves
- Cuffs made of elastic jersey
- Latex-free
- Sterile, for single use
- Comes in 3 sizes: Large, XLarge and XXLarge.
- Length (shoulder seam to mid calf):
- size Large: ± 140 cm
- size XLarge: ± 150 cm
- size XXLarge: ± 160 cm
Packaging & Labelling
- Unit presentation with reverse folding in order to ensure an aseptic gowning technique
- Wrapped in a drape
- Double sterile packaging in peel-open pack
Instructions for use
Disposable sterile gowns are intended for situations where sterilization is problematic. It is recommended to keep a small emergency stock in every hospital.
Single use linen is MSF standard for orthopedic surgical procedures.
Surgeries at high risk (= high performance level needed):
- any procedure in which the surgeons hands are in a body cavity
- orthopedic procedures during which tourniquets are not used
- open cardiovascular or thoracic procedures
- trauma procedures
- C-sections
Precautions for Use
According to the quality standards, the back of the surgical gown may have a low liquid penetration barrier performance: can be ANSI/AAMI-Level 1 even though the whole gown is classified as ANSI/AAMI-Level 4 surgical gown. Therefore, using a surgical gown in isolation settings may not provide appropriate protection.




![[KMEDMEBO17A] (module VHF) SAMPLING AND SMALL ISOLATION](/web/image/product.template/571758/image_256/%5BKMEDMEBO17A%5D%20%28module%20VHF%29%20SAMPLING%20AND%20SMALL%20ISOLATION?unique=72f3843)
![[KMEDMHOS36-] (mod OT Room) SURGICAL LINEN SINGLE USE 2021](/web/image/product.template/574360/image_256/%5BKMEDMHOS36-%5D%20%28mod%20OT%20Room%29%20SURGICAL%20LINEN%20SINGLE%20USE%202021?unique=0aa6d32)