SURGICAL HOOD, non-woven, s.u.
STD
ELINHOOS1--
Valid Article
Account code:
60210
HS Code:
903300
Last Updated on:
29/06/2025, 22:40:15
Former
Code(s):
-X
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
T0207 - Caps and headwear (excluding personal protective equipment - ppe)
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
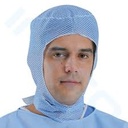
SURGICAL HOOD, non-woven
Definition
A non-sterile head covering designed as a top with elongated sides and back with a front opening for the face to be used by surgical staff to completely cover their hair in order to protect the patient from micro-particles falling into the surgical field during a surgical intervention.
This is intended for projects in which internal fixation is being performed. It is a single-use device.
Specifications
Technical specifications
- Non-woven fabric: 25 g/m2
- Air permeable
- Complete head coverage leaving only the face uncovered
- Wide ties, for closure around the neck
- Colour: green or blue
- Universal size
- Non sterile, for single use
Packaging & Labelling
Box of 50 or 100 pieces
MSF requirements
The wearing of a surgical hood by the operating theatre staff is part of the minimal criteria when performing internal fixation in surgical orthopaedic programmes.
Some restricted information has been hidden. Sign in
to see this information