CURETTE, BONE, VOLKMANN, 17 cm 13 mm, 29-51-55
STD
ESURCUBV13-
Valid Article
Account code:
60100
HS Code:
901890
Last Updated on:
13/11/2025, 22:09:59
Former
Code(s):
-X
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
L090901 - Bone cutters, reusable
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
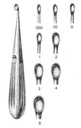
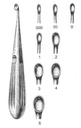
Bone curette, Volkmann
Definition
A manual surgical instrument designed for cutting and excising bone tissue typically during an orthopaedic or a plastic surgery procedure.
It is typically designed as a long, slender instrument with a handle at the proximal end and a concave, spoon-like tip which has a sharp edge, at the distal end. It is used to facilitate the removal of the bone tissue without causing trauma to the surrounding muscles.
Specifications
Technical specifications
- Bone curette, hollow spoon-shaped
- Cutting edges
- Length: 17 cm
- Material: martensitic steel
| code | size | width |
| ESURCUBV03- | 000 | 3.6 mm |
| ESURCUBV04- | 00 | 4.4 mm |
| ESURCUBV06- | 1 | 6.8 mm |
| ESURCUBV08- | 2 | 8.5 mm |
| ESURCUBV10- | 3 | 10 mm |
| ESURCUBV13- | 5 | 13 mm |
Some restricted information has been hidden. Sign in
to see this information





![[KSURBBON42I] BONE SET, inferior limbs, 40 instruments](/web/image/product.template/569757/image_256/%5BKSURBBON42I%5D%20BONE%20SET%2C%20inferior%20limbs%2C%2040%20instruments?unique=d3b1900)
![[KSURBBON14-] BONE SET, inferior + superior limbs, 14 instruments](/web/image/product.template/569760/image_256/%5BKSURBBON14-%5D%20BONE%20SET%2C%20inferior%20%2B%20superior%20limbs%2C%2014%20instruments?unique=6db9c46)