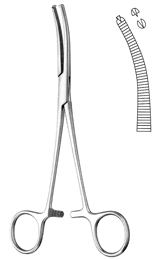
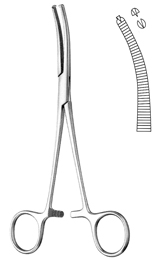
FORCEPS, HAEMOST. R-OCHSNER, 16 cm, curved 1x2teeth 16-13-16
STD
ESURFHRO16C
Valid Article
Account code:
60100
HS Code:
901890
Last Updated on:
29/06/2025, 22:44:16
Former
Code(s):
-X
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
L031306 - Hemostatic forceps, reusable
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Haemostatic forceps, Rochester-Ochsner
Definition
Pressure force instrument and springs: haemostatic forceps.
Multipurpose in surgery: haemostasis, dissection, gripping of vessels.
R-Ochsner = Rochester-Ochsner
Specifications
Technical specifications
- Curved haemostatic forceps
- 1 x 2 teeth
- Heavily transversal serrated jaws
- Flexible arms
- Progressive setting and locking of the ratchet
- Adjustment of the teeth
- Length: 16 cm
- Material: martensitic steel
Some restricted information has been hidden. Sign in
to see this information