NASAL OXYGEN CANNULA, 2 prongs + tube, premature
STD
SCTDCANN2PL
Valid Article
Account code:
60210
HS Code:
901890
Last Updated on:
26/02/2025, 22:01:18
Former
Code(s):
-X
Single patient multiple use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
R03010203 - Oxygen therapy nasal cannulas
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
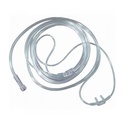
NASAL CANNULA, OXYGEN
Definition
A non-sterile, semi-rigid tube with nasal prongs designed to be inserted into the nostrils of a patient, and held in place with an over-the-ear tubing, to administer oxygen.
Synonym
nasal prongs, over-the-ear cannula
Specifications
Quality standards
- EN 13544-2, 2002, +A1 2009 Respiratory therapy equipment. Tubing and connectors
- ISO 15001, 2010, edition 2, (confirmed 2022) Anaesthetic and respiratory equipment — Compatibility with oxygen
- ISO 18190, 2016, edition 1, Anaesthetic and respiratory equipment - General requirements for airways and related equipment
Technical specifications
- PVC low resistance tubing, round shape section, designed for low-flow procedures (0-15l/min)
- Tubing compatibility with standard oxygen connecting tubing, 3-5mm internal diameter and 7-8mm external diameter, and 15/22 mm diameter ventilation tubing. Multi-channel design which permits oxygen flow even if the tubing is kinked.
- Narrow, atraumatic (curved) prongs with lateral eyes, fixed to a flexible horizontal support
- Over-the-ear tubing adjustable using a sliding ring
- Y connection with oxygen tube fitted with female conical connector
- Maximum flow/ minute (according the Ø of delivery tube)
- premature and neonates: 1.5 - 2 l/min
- child and adult: 6 liter/min
- Non sterile, for single patient use
- Comes in 4 sizes: adult, child, neonate, premature
- Length of the oxygen tube: ± 2 m
Packaging & Labelling
Individually packed in a sealed plastic envelope
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Some restricted information has been hidden. Sign in
to see this information




![[KMEDMHDS21-] (mod delivery & neonate) RENEWABLE SUPPLIES compl. 2019](/web/image/product.template/569223/image_256/%5BKMEDMHDS21-%5D%20%28mod%20delivery%20%26%20neonate%29%20RENEWABLE%20SUPPLIES%20compl.%202019?unique=2ba5e0d)
![[KMEDMHES35-] (mod emergency) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574355/image_256/%5BKMEDMHES35-%5D%20%28mod%20emergency%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=54efbab)