SUPRAPUBIC PUNCTURE SET, CH10, adult, sterile, s.u.
Valid Article
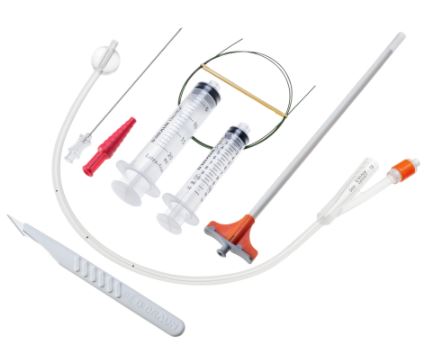
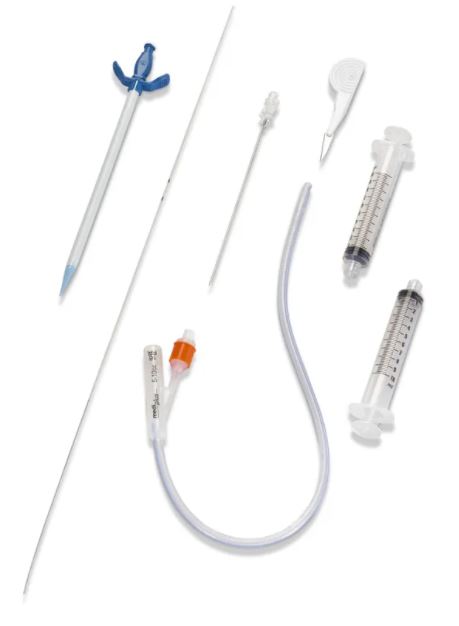
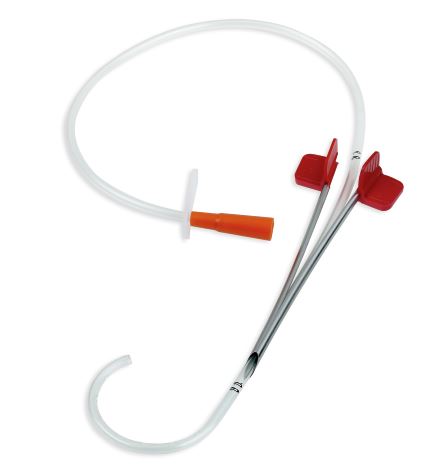
SUPRA-PUBIC CATHETER PUNCTURE SET
Definition
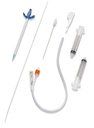
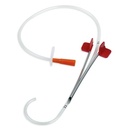
A sterile, flexible/semi-rigid tube intended to be inserted through a suprapubic incision directly into the bladder of patient primarily for the drainage of urine. It consists of a straight single-lumen tube. The proximal end remains external to the patient and is connected to a urine collection device. It includes a trocar to assist in its insertion.
Specifications
Quality standards
Technical specifications
- Drainage catheter:
- polyurethane (short and medium care) or silicone (long time care)
- pigail- tip (J-tip) for minimum irritation of bladder
- open distal end with lateral eyes
- with clamp or plug
- funnel shape connector for connecting to the bag
- selected size: CH 10
- length: ± 50 cm (40 - 70 cm)
- Trocar = introducer:
- stainless steel, cleavable (option = trocar in cannula that splits in two parts)
- length: 8 to 12 cm
- Sterile, for single use
Packaging & Labelling
Unit sterile packaging in peel-open pack
To be Ordered Separately
Articles supplied with the puncture set are optional as available in health facilities where this procedure is carried out:
- Urine bag:
- PVC
- graduated
- capacity: 1 to 2 litres
- Fixation device (surgical tape or fixation disc) to hold the catheter firmly in place on the skin
- scalpel
These articles may be included or not, depending on the manufacturer.
Instructions for use
Precautions for Use
Available suprapubic catheter kits vary considerably in their composition. Read carefully the package insert that comes with the kit. Check for exact content, some articles are optional and have to be added from the hospital stock.
Immediately after use, dispose of the trocar into a sharps container (see related articles below).
(Cf Public Health Engineering In Precarious Situations, MSF, 2010, Chapter 6)



![[SCTDBAGU2VSS] URINE BAG 2 l, drain., non-ret. valves, sampling port, ster.](/web/image/product.template/571478/image_256/%5BSCTDBAGU2VSS%5D%20URINE%20BAG%202%20l%2C%20drain.%2C%20non-ret.%20valves%2C%20sampling%20port%2C%20ster.?unique=a700e1e)
![[SINSCONT1R-] REUSABLE SHARPS CONTAINER (RSC), 1.2 litre](/web/image/product.template/569744/image_256/%5BSINSCONT1R-%5D%20REUSABLE%20SHARPS%20CONTAINER%20%28RSC%29%2C%201.2%20litre?unique=248fc0c)
![[SINSCONT4P-] SHARPS CONTAINER, 4 l, plastic, s.u.](/web/image/product.template/569672/image_256/%5BSINSCONT4P-%5D%20SHARPS%20CONTAINER%2C%204%20l%2C%20plastic%2C%20s.u.?unique=ed6ba12)
![[KMEDMHOS45-] (mod OT Room) CATHETERS AND DRAINS 2021](/web/image/product.template/574392/image_256/%5BKMEDMHOS45-%5D%20%28mod%20OT%20Room%29%20CATHETERS%20AND%20DRAINS%202021?unique=abfc26b)