TUBE, ENDOTRACHEAL, s.u. + CONN., w/o balloon, Ø 2.5 mm
Valid Article
BASIC ENDOTRACHEAL TUBE, non cuffed
Definition
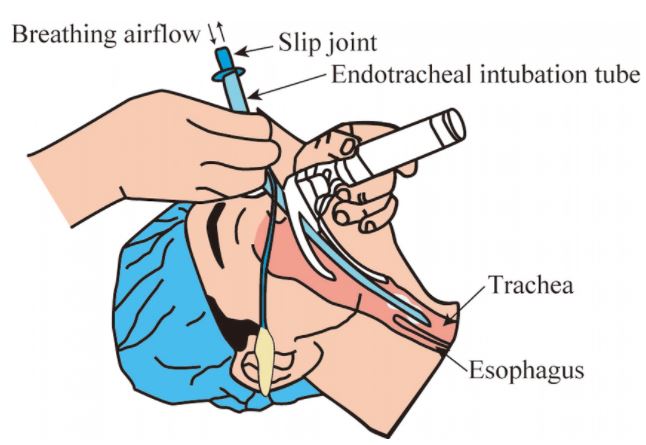
The endotracheal tube is a tube that is placed in the trachea through the mouth or nose and between the vocal cords. It serves to provide oxygen and inhaled gases to the lungs and protects the lungs from contamination, such as gastric contents or blood. It secures the airway and permits manual or mechanical ventilation in anaesthesia or resuscitation procedures.
Specifications
Quality standards
Technical specifications
Tracheal tube with open distal end and Magill-type point with oro-nasal angle of 37.5°
- preformed tube: the curvature of the ETT is called "the Magill Curve", it is a curvature of +/- 140 mm radius, +/- 20 mm.
- transparent PVC
- size marked on the tube (inner diameter in mm)
- graduated every cm
- open distal end with atraumatic 38º bevel, open on the left
- Murphy eye is optional
- proximal end with standard connector, 15 mm outer Ø, for connecting to the ventilation system
- radio-opaque line to confirm correct placement
- uncuffed
- Sterile, for single use
Packaging & Labelling
Sterile unit packaging: peel-off sachet.
Instructions for use
Placing of an endotracheal tube should be done by skilled staff.
It requires the use of accessories (laryngoscope, Magill forceps...), as well as specific equipment for manual ventilation and tracheal suction.





![[EANECAME01-] CAPNOGRAPH, handheld, battery (Emma) mmHg](/web/image/product.template/575491/image_256/%5BEANECAME01-%5D%20CAPNOGRAPH%2C%20handheld%2C%20battery%20%28Emma%29%20mmHg?unique=f85f0fe)
![[EANEFOMA1P-] FORCEPS, MAGILL, paediatric, 15 cm](/web/image/product.template/569488/image_256/%5BEANEFOMA1P-%5D%20FORCEPS%2C%20MAGILL%2C%20paediatric%2C%2015%20cm?unique=93482d1)
![[EANELARY7--] LARYNGOSCOPE FO (fibreoptic) + 7 blades](/web/image/product.template/569659/image_256/%5BEANELARY7--%5D%20LARYNGOSCOPE%20FO%20%28fibreoptic%29%20%2B%207%20blades?unique=9688a7d)
![[SCTDINTAB06] ENDOTRACHEAL TUBE INTRODUCER, sterile, s.u., CH06, 50 cm](/web/image/product.template/572104/image_256/%5BSCTDINTAB06%5D%20ENDOTRACHEAL%20TUBE%20INTRODUCER%2C%20sterile%2C%20s.u.%2C%20CH06%2C%2050%20cm?unique=5d558c3)
![[SCTDINTAB10] ENDOTRACHEAL TUBE INTRODUCER, sterile, s.u., CH10, 70 cm](/web/image/product.template/572105/image_256/%5BSCTDINTAB10%5D%20ENDOTRACHEAL%20TUBE%20INTRODUCER%2C%20sterile%2C%20s.u.%2C%20CH10%2C%2070%20cm?unique=5d558c3)
![[SMSULUBRW05S] LUBRICANT, water based, sterile, unidose, 5g](/web/image/product.template/570124/image_256/%5BSMSULUBRW05S%5D%20LUBRICANT%2C%20water%20based%2C%20sterile%2C%20unidose%2C%205g?unique=ef93e4d)
![[KMEDMHES35-] (mod emergency) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574355/image_256/%5BKMEDMHES35-%5D%20%28mod%20emergency%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=8014f55)
![[KMEDMHOS45-] (mod OT Room) CATHETERS AND DRAINS 2021](/web/image/product.template/574392/image_256/%5BKMEDMHOS45-%5D%20%28mod%20OT%20Room%29%20CATHETERS%20AND%20DRAINS%202021?unique=abfc26b)