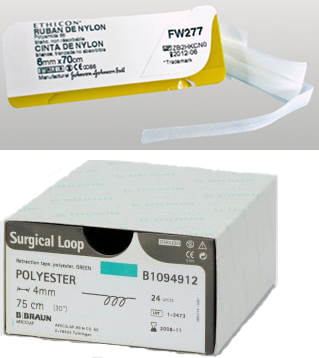
SURGICAL VESSEL LOOP, 4 mm, 60 cm
STD
SSUTSLOO4B6
Valid Article
Account code:
60210
HS Code:
901890
Last Updated on:
29/06/2025, 23:17:33
Former
Code(s):
-X SSUTTASU4B6
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
H900202 - Marking tapes, vascular structures
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
SURGICAL VESSEL LOOP
Definition
A sterile, nonimplantable ribbon intended to be used during a surgical procedure to assist a surgeon to identify, retract and occlude blood vessels, tendons and nerves. It is constructed of synthetic polymer and is typically coloured to facilitate visualization.
Specifications
Quality Standards Comment
European Pharmacopoeia
Technical specifications
- Synthetic: silicone, polyester, polypropylene, nylon ... (latex-free). cotton is not an interesting material as it absorbs liquids
- Tubular (preferred) or braided
- Non-absorbable
- Width: 3 to 4 mm or 2 - 2.5 mm
- Length: +/- 60 cm
- No colour requirements
- Sterile, for single use
Packaging & Labelling
Unit double sterile packaging
Box of 15 or 24 units, according to manufacturer
Instructions for use
Suspensory tape only used during the surgical intervention to isolate vessels or nerves, and removed before closure of the operation wound.
Storage
Below 30°C
Some restricted information has been hidden. Sign in
to see this information
Some restricted information has been hidden. Sign in
to see this information