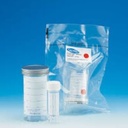
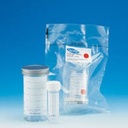
CONTAINER, SAMPLE, double packaging, 100 ml, sterile
STD
STSSCONT100SD
Valid Article
Account code:
60200
HS Code:
382213
Last Updated on:
29/06/2025, 23:44:36
Former
Code(s):
ELAEZBD0386 ELABCONT100SD
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
W05020102 - Samples transport, boxes
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
SAMPLE CONTAINER, sterile, double packaging
Definition
Transparent container with screw cap, used for biological sample collection in operation theatre and transport to an external laboratory.
Specifications
Some restricted information has been hidden. Sign in
to see this information
Material
Container in polystyrene (PS) and cap in polypropylene (PP).
Technical specifications
- Sterile container inside and outside, internal packaging sterile. Sterilised by gamma irradiation.
- Double packaging unit in peelable bag.
- Volume 100 ml.
- h x Ø: 76 mm x 50 mm
- Screw cap
- Single use
- Leak-tested
- Printed label
Packaging & Labelling
Instructions for use
Useful for the transport of samples from the OT to the laboratory or for the transportation to an external laboratory.
Storage
Shelf live: 36 months.
MSF requirements
Reserved for bacteriology programmes.
Some restricted information has been hidden. Sign in
to see this information