WRIST ORTHOSIS, immobilization, medium, right
STD
EPHYWROR1M-
Valid Article
Account code:
60210
HS Code:
902110
Last Updated on:
22/08/2025, 22:01:23
Former
Code(s):
EPHYZFR0031
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
Y060609 - Wrist (forearm) orthoses
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
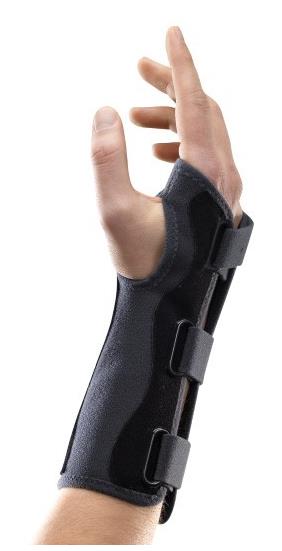
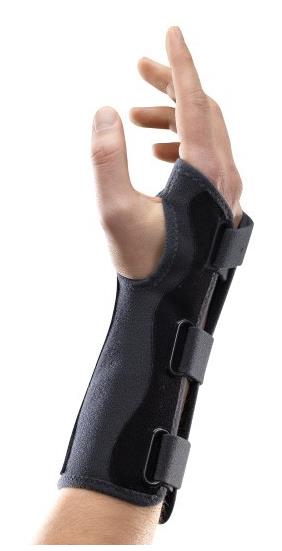
WRIST ORTHOSIS
Definition
An externally applied orthopaedic appliance or apparatus designed to support, align, prevent, or correct deformities/injuries or to improve function of the wrist. This is a reusable device.
Synonym
wrist splint
Specifications
- Wrist orthosis that fits wrist and thumb
- Model without thumb support / immobilization
- With aluminium anterior plate to maintain 45° extension of wrist
- Radiotranslucent
- Velcro straps on posterior
- Soft, resistant and washable material
- Adult medium size (variations possible according the manufacturer): Ø wrist: >18 à 22 cm
Instructions for use
- Measure wrist circumference to choose the size.
- The wrist has to be placed with 20° to 30° of dorsi-flexion (bend the reinforcement bars in proportion).
- The orthosis well placed allows a full flexion and extension of the fingers.
Some restricted information has been hidden. Sign in
to see this information
Precautions for Use
Remove the reinforcement bars if possible.
Maintenance
Follow the manufacturer's indications for cleaning.
MSF requirements
For people with wrist pain or following wrist trauma.
All contexts, more particularly with injured patients.
Some restricted information has been hidden. Sign in
to see this information