SUTURE REMOVAL SET, disposable
STD
SSURSUTR01S
Valid Article
Account code:
60210
HS Code:
300590
Last Updated on:
13/11/2025, 23:18:21
Former
Code(s):
SDREZTF0038 SDRESDRS01S SDRESSRS01S SSURSUTR03- SDREZFR0055 SDRESSRS03-
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
K01020120 - Minimally invasive surgery surgical instrument kits, single-use
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
SUTURE REMOVAL SET, disposable
Definition
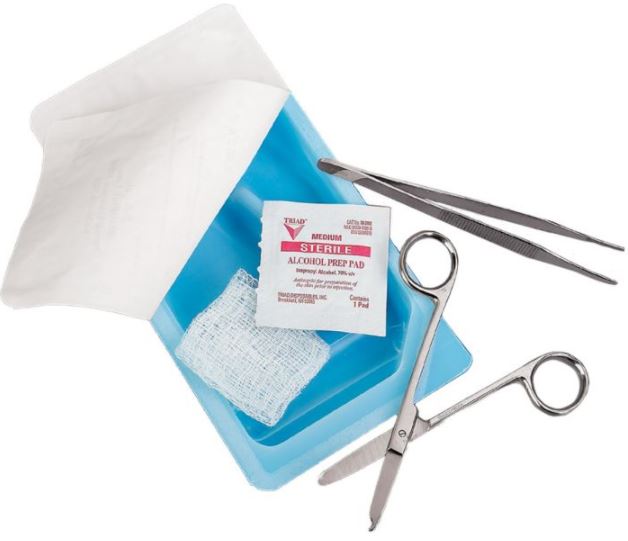
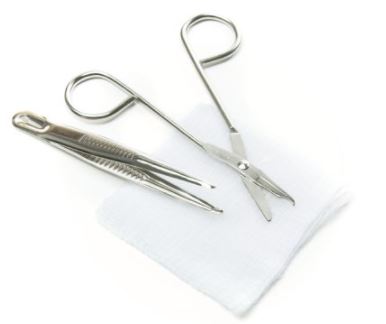
A collection of various sterile instruments and dressings designed to remove sutures from a patient. It typically includes scissors, forceps, gauze and bandages. This is a single-use device.
Specifications
Proposed content (Tetra 13463)
- 5 x non woven compresses 7.5 x 7.5 cm
- 1 x single use tweezer forceps 13 cm
- 1 x stainless steel thread cutter, long for single use
Sterile, for single use
Packaging & Labelling
Single set in sterile peel open pack (blister)
Instructions for use
Precautions for Use
Sterility is only guaranteed if the blister is intact and the product is only opened immediately before use.
- Check the integrity of the set before use.
- To be opened only immediately before use.
- Do not re-use or re-sterilise the instruments
MSF requirements
Supplied with the skin biopsy set and is included in the VHF kit.
Some restricted information has been hidden. Sign in
to see this information