LARYNGOSCOPE FO (fibreoptic) + 7 blades
Valid Article
FIBREOPTIC LARYNGOSCOPE + 7 blades
Definition
A hand-held device intended to be used by anaesthesia/emergency service personnel to manipulate the tongue, preventing it from obstructing the oropharynx and enabling a clear view of the trachea for the insertion of an endotracheal tube prior to the delivery of inhalation anaesthesia and/or ventilation.
It has a handle containing batteries to power its fibreoptic light for airway illumination, and a curved or straight blade of various lengths that can be hinged/interchanged.
Specifications
Quality standards
Technical specifications
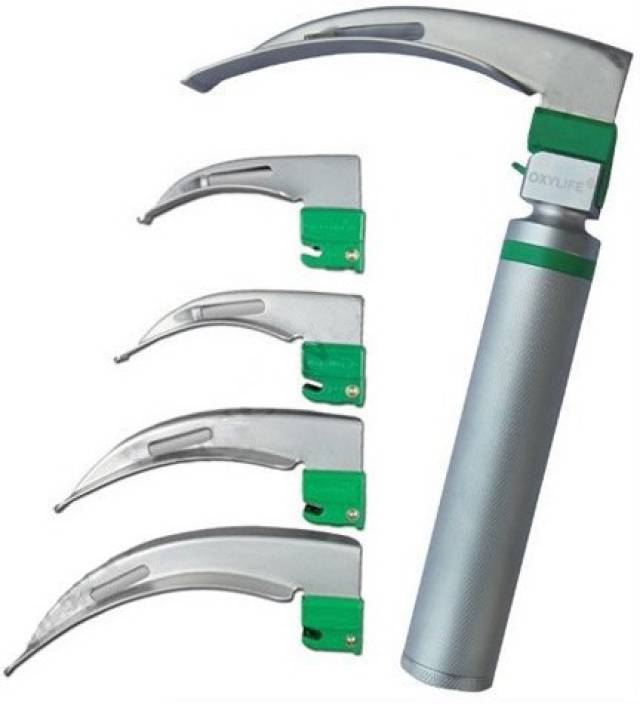
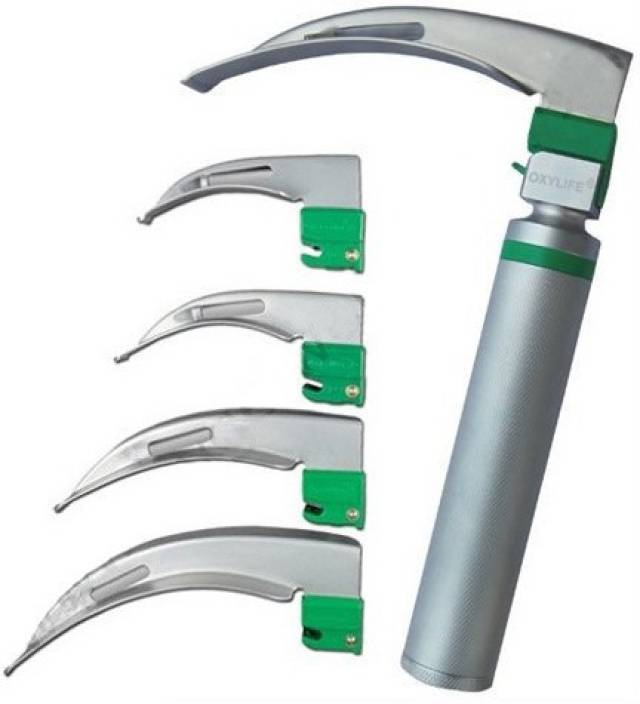
Rigid case including 2 handles and 7 blades
HANDLE
- 2 sizes:
- 1 handle diameter 28 mm
- 1 handle diameter 19 mm, for better ergonomy while using small blades
- chromium-plated brass or stainless steel
- hollow and cylindrical
- the handle of 28 mm is fitted for 2 x R14 batteries 1.5V, the handle of 19 mm for 2 x AA batteries, alcaline, 1.5V
- fitted with a bulb, 2.5 V which switches on when the handle and the blade are connected
- end with contact pads fitting the various blades
- with green coloured ring, normalised (FO)
BLADES
- stainless steel
- fibre optic = FO (transmitting the light to the tip of the blade)
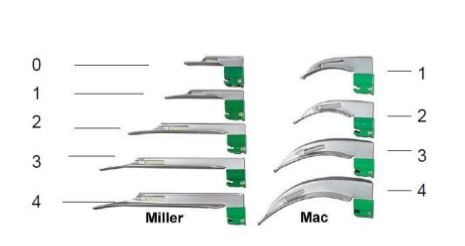
- 6 curved Macintosh blades FO:
- 1 x nº 0, total length 80 mm (± 5 mm): for infants from 6 to 12 months
- 1 x nº 1, total length 90 mm (± 5 mm): for toddlers from 1 to 2 years
- 1 x nº 2, total length 110 mm (± 5 mm): for children from 2 to 10 years
- 2 x nº 3, total length 130 mm (± 5 mm): for children > 10 years and small adults
- 1 x nº 4, total lenght 150 mm (± 5 mm): for tall adults
- 1 straight Miller blade, nº 0, total length 78 mm (± 5 mm): for new-borns and infants up to 6 months
Supplied with the Article
- 2 x R14 batteries 1.5 V or 2 x AA batteries, alcaline, 1,5V
- 5 x spare-bulbs (only 1 spare if LED)
Instructions for use
Precautions for Use
To prevent batteries from deteriorating, it is recommended to remove them from the handle when the instrument is unused for a prolonged period or stored under damp conditions.
Maintenance
After each use:
- The blades must be cleaned, pre-disinfected and sterilized by steam autoclave (caution, repeated sterilization decreases the quality of the light transmitted by the fibres).
- The handle must be cleaned and disinfected. Use a detergent/disinfectant for surfaces/non-invasive medical equipment. Prepare use-solution in concentration required. Always read the label and product information before use. Do not mix the with other products. Make sure to wet surfaces completely, use a wipe or towel, keep them wet for the whole exposure time.
(Cf Introduction: Disinfection and sterilization in the field)
MSF requirements
This laryngoscope forms part of the emergency anaesthesia equipment and must always be present and kept ready for use in all hospital settings.








![[KMEDMHIE21-] (mod ICU) EXAMINATION-RESUSCITATION EQUIPMENT](/web/image/product.template/574340/image_256/%5BKMEDMHIE21-%5D%20%28mod%20ICU%29%20EXAMINATION-RESUSCITATION%20EQUIPMENT?unique=cb394cc)
![[KMEDMHOE17-] (mod OT Room) ANESTHESIA-RESUSCITATION EQ.](/web/image/product.template/572539/image_256/%5BKMEDMHOE17-%5D%20%28mod%20OT%20Room%29%20ANESTHESIA-RESUSCITATION%20EQ.?unique=dcf247b)
![[KMEDMHAE221] (mod AMP) COMPLEMENTARY RESUSCITATION EQUIPMENT](/web/image/product.template/572665/image_256/%5BKMEDMHAE221%5D%20%28mod%20AMP%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT?unique=c0e1972)
![[KMEDMHEE321A] (mod emergency) COMPLEMENTARY RESUSCITATION EQUIPMENT 2021](/web/image/product.template/574367/image_256/%5BKMEDMHEE321A%5D%20%28mod%20emergency%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT%202021?unique=9bb058c)
![[EANELABL0CF] (laryngoscope) BLADE MAC INTOSH nº 0, curved, optic fibre](/web/image/product.template/569941/image_256/%5BEANELABL0CF%5D%20%28laryngoscope%29%20BLADE%20MAC%20INTOSH%20n%C2%BA%200%2C%20curved%2C%20optic%20fibre?unique=39de8d0)
![[EANELABL1CF] (laryngoscope) BLADE MACINTOSH nº1, curved, fiber optic](/web/image/product.template/569478/image_256/%5BEANELABL1CF%5D%20%28laryngoscope%29%20BLADE%20MACINTOSH%20n%C2%BA1%2C%20curved%2C%20fiber%20optic?unique=93482d1)
![[EANELABL1SF] (laryngoscope) BLADE MILLER nº0, str., neonatal, fiber optic](/web/image/product.template/569485/image_256/%5BEANELABL1SF%5D%20%28laryngoscope%29%20BLADE%20MILLER%20n%C2%BA0%2C%20str.%2C%20neonatal%2C%20fiber%20optic?unique=60039db)
![[EANELABL2CF] (laryngoscope) BLADE MACINTOSH nº2, curved, fiber optic](/web/image/product.template/569398/image_256/%5BEANELABL2CF%5D%20%28laryngoscope%29%20BLADE%20MACINTOSH%20n%C2%BA2%2C%20curved%2C%20fiber%20optic?unique=60039db)
![[EANELABL3CF] (laryngoscope) BLADE MACINTOSH nº3, curved, fiber optic](/web/image/product.template/569396/image_256/%5BEANELABL3CF%5D%20%28laryngoscope%29%20BLADE%20MACINTOSH%20n%C2%BA3%2C%20curved%2C%20fiber%20optic?unique=93482d1)
![[EANELABL4CF] (laryngoscope) BLADE MACINTOSH nº4, curved, fiber optic](/web/image/product.template/569397/image_256/%5BEANELABL4CF%5D%20%28laryngoscope%29%20BLADE%20MACINTOSH%20n%C2%BA4%2C%20curved%2C%20fiber%20optic?unique=60039db)
![[EANELARBFO1L] (laryngoscope, RIESTER, FO) LED BULB, spare 2.5V](/web/image/product.template/587186/image_256/%5BEANELARBFO1L%5D%20%28laryngoscope%2C%20RIESTER%2C%20FO%29%20LED%20BULB%2C%20spare%202.5V?unique=a32c975)
![[PELEBATTA06] BATTERY dry cell (R6/AA) alkaline, 1.5V](/web/image/product.template/549279/image_256/%5BPELEBATTA06%5D%20BATTERY%20dry%20cell%20%28R6-AA%29%20alkaline%2C%201.5V?unique=660d3f9)
![[PELEBATTR14] BATTERY rechargeable (R14/C) NiMH, 1.2V](/web/image/product.template/549282/image_256/%5BPELEBATTR14%5D%20BATTERY%20rechargeable%20%28R14-C%29%20NiMH%2C%201.2V?unique=489269c)