SPLINT of BOHLER-BRAUN, adjustable femur length
Valid Article
SPLINT of Bohler/Braun
Definition
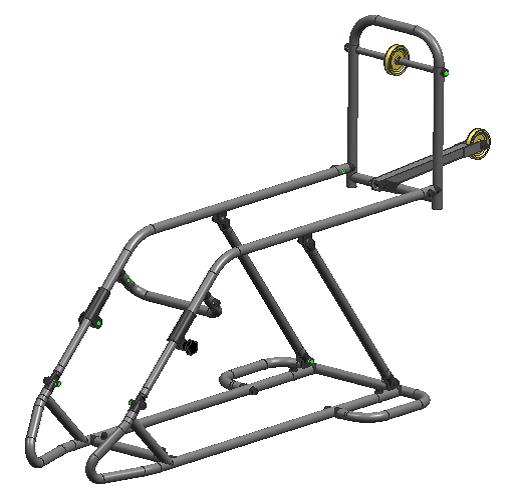
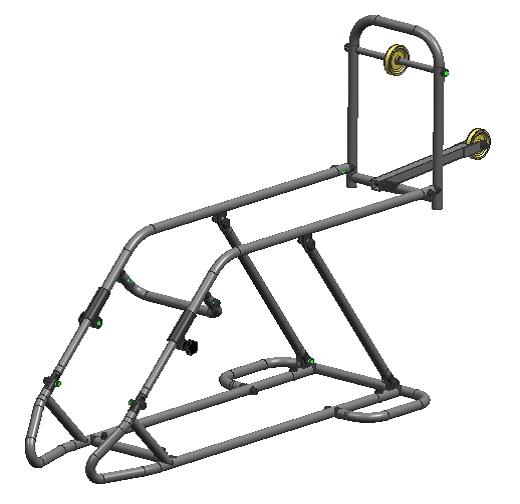
It is a common splint used in wards (placed on a patient's bed) for immobilization and reduction of most lower limb fractures and treatment of other lower limb pathologies. It consists of a steel tubing structure with a 2 pulleys for application of mobile traction. (It is modified from Braun splint which consists of only one pulley.)
Replaces the Boppe splint.
Synonym
ICRC splint
Specifications
Technical specifications
- Material: Stainless steel and plastic
- Steel tube frame (D20x1.0mm, St52-3, DIN2394, welded; D17x1.0mm, St, DIN2394, welded)
- Powder coating ~40μm on all surfaces except hinge faces
- Parts connected with hexagonal socket bolts M8 (ISO4762) and Hexagonal socket bolts with shaft M6/D8 (ISO 7379)
- Extensible and dismountable splint
- 7 positions with the adjustable pulley height femur traction
- Allen key to adjust the pulley angle tibial traction
- 6 pieces and 8 bolts (dis)mountable with Allen key
- Adjustable femur support : 390 - 500 mm
- Length tibia support : 580 mm
- Width (between tubes) : 208 mm
- 2 Pulley positions: 23° (femur) and 0° (tibia)
- Counter weight : max 15 kg
- Adult model, dimensions when dismantled:
- length: 850 mm
- width: 120 mm
- height: 300 mm
- weight : 10 kg
Packaging & Labelling
Supplied dismantled with assembly diagram.
Instructions for use
Used for treating femur or tibia fractures in adults by traction-suspension.
Order 2 or 3 splints for a 100-bed hospital.
Precautions for Use
Test the stability of the device (supported on the bed) before installing the patient.
Maintenance
It is important to clean the frame regularly and certainly when removed from the patient. All the parts are made of stainless steel.The parts can be washed with a damp cloth.
MSF requirements
Essential item for treating the femur or tibia fractures where orthopaedic surgery is not possible, especially in isolated rural hospitals.
Optional article of the Ward, medical equipment part of the modular hospital kit.



![[KMEDKHOE2CO] OPERATING THEATRE, PART med.equip. 1operating room, complete](/web/image/product.template/572548/image_256/%5BKMEDKHOE2CO%5D%20OPERATING%20THEATRE%2C%20PART%20med.equip.%201operating%20room%2C%20complete?unique=dcf247b)
![[KMEDKHWE1CO] WARD, PART medical equipment ward 20-40 beds, complete](/web/image/product.template/573288/image_256/%5BKMEDKHWE1CO%5D%20WARD%2C%20PART%20medical%20equipment%20ward%2020-40%20beds%2C%20complete?unique=412660e)