INCENTIVE SPIROMETER, portable, single patient, adult
STD
EPHYSPIR1A-
Valid Article
Account code:
60210
HS Code:
901890
Last Updated on:
30/06/2025, 00:20:17
Former
Code(s):
EPHYZFR0077 EPHYZBE0023
Single patient multiple use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
Z121501 - Spirometry instruments
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
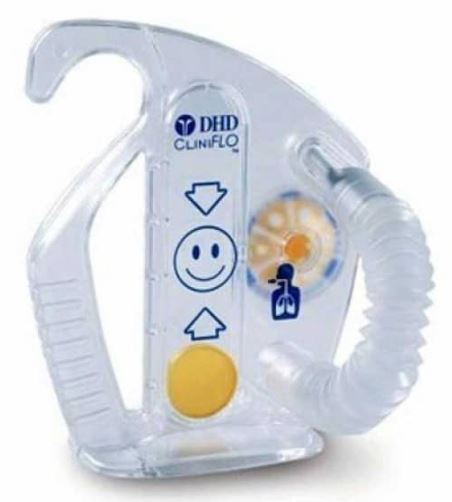
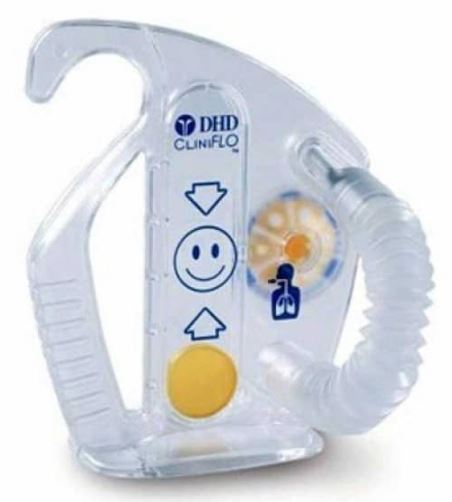
INCENTIVE SPIROMETER
Definition
A portable mechanical breathing-muscle exerciser used in respiratory therapy to encourage and motivate deep-breathing manoeuvres, typically for the postsurgical treatment and prevention of atelectasis (lung collapse) and to help facilitate airway opening and clearing.
It includes an indicator in a housing that rises in relation to patient inspiratory effort, performed via an attached tube and mouthpiece, to determine the maximum volume of air which can be inspired.
Specifications
Quality standards
EN ISO 26782, 2009, (confirmed 2020) Anaesthetic and respiratory equipment - Spirometers intended for the measurement of time forced expired volumes in humans
Technical specifications
- Plastic bottle/container, latex-free
- Minimum volume/capacity:
- adult: 4 litres
- child/elderly person: 2.5 litres
- Flexible tubing connected to the container
- Mouth piece at distal end of tubing
- One-way valve to ensure that patients inhale, rather than exhale into the unit
- Colored highly visible and moveable piston
- Universal graphical graduation (indicating correct inspiratory flow rate)
- External adjustable inspiratory indicator attached to the container
- Single Patient Use
Instructions for use
The patient must breathe in slowly and as deeply as possible.
MSF requirements
Rehabilitation item used in all types of contexts.
Some restricted information has been hidden. Sign in
to see this information

![[KMEDMHWE36-] (mod ward) PHYSIOTHERAPY, traumatology, 50 persons 2021](/web/image/product.template/574568/image_256/%5BKMEDMHWE36-%5D%20%28mod%20ward%29%20PHYSIOTHERAPY%2C%20traumatology%2C%2050%20persons%202021?unique=6cf1e52)
![[KMEDMHIE21-] (mod ICU) EXAMINATION-RESUSCITATION EQUIPMENT](/web/image/product.template/574340/image_256/%5BKMEDMHIE21-%5D%20%28mod%20ICU%29%20EXAMINATION-RESUSCITATION%20EQUIPMENT?unique=7433b39)
![[KMEDMHWE33-] (mod ward) MEDICAL EQUIPMENT 2021](/web/image/product.template/574356/image_256/%5BKMEDMHWE33-%5D%20%28mod%20ward%29%20MEDICAL%20EQUIPMENT%202021?unique=010fcc3)