FILM DRESSING, semi-permeable, adhesive, IV, sterile, L
Valid Article
FILM DRESSING, semi-permeable, IV
Definition
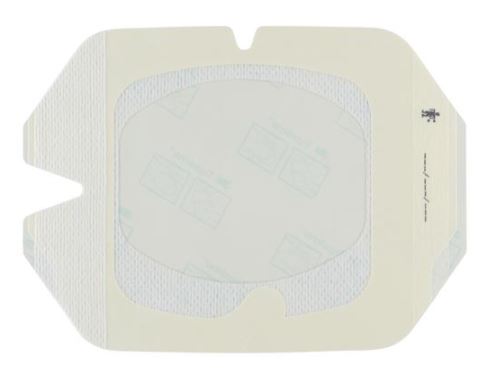
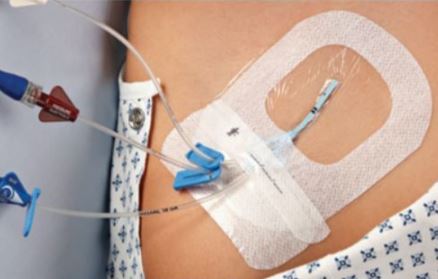
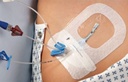
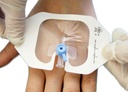
A sterile, transparent, adhesive and semi-permeable (i.e. impermeable to fluids, permeable to vapours and gases) covering. It is applied to secure to skin vascular catheters, or infusion ports and is intended to provide protection to fluids and external contaminants.
Specifications
Quality standards
Technical specifications
- Transparent film made of synthetic polymer (e.g., polyurethane based), latex- and PVC-free
- Breathable: allows moisture vapor and gas exchanges
- Moisture Vapour Transmission Rate ≥ 500g/m²/24h
- Waterproof: impervious to liquids and body fluids
- Protection against all external contamination
- Barrier against bacteria and viruses ≥20nm
- Adhesive:
- must adhere to the skin during 7 days minimum
- hypoallergenic latex-free adhesive
- With frame for easy positioning
- Documentation tape strip : preprinted for documenting dressing changes (optional)
- Securement tape strip to enhance stabilization of the catheter (optional but practical)
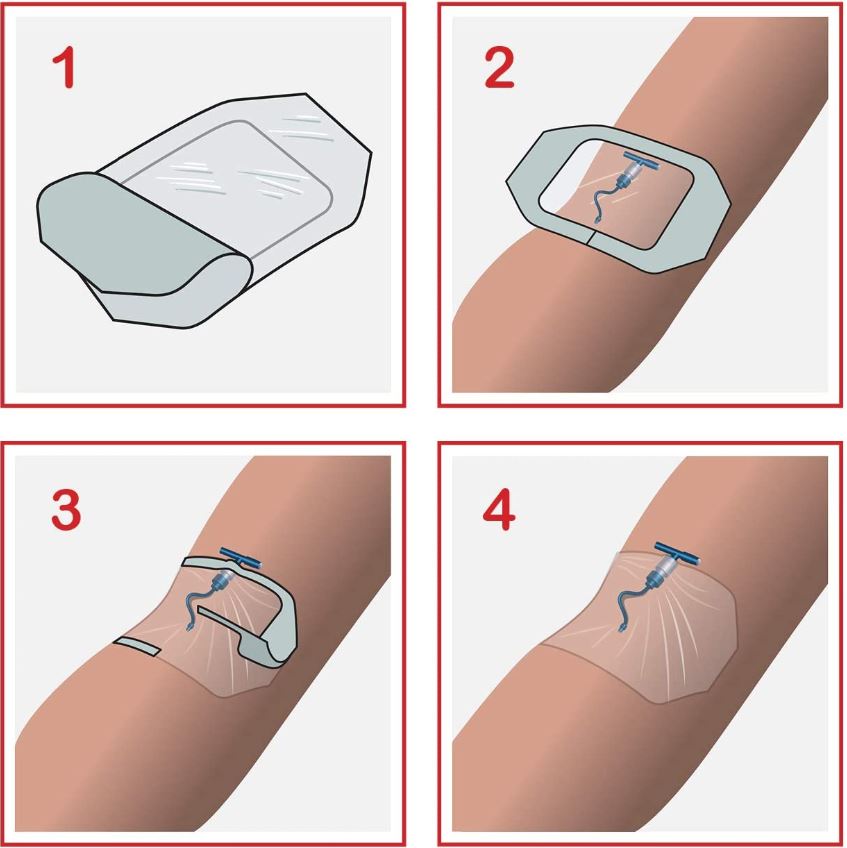
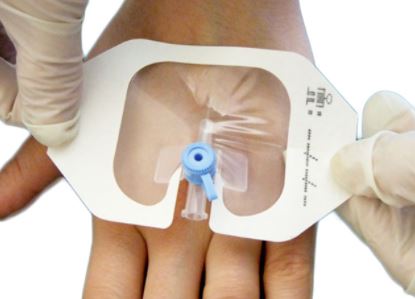
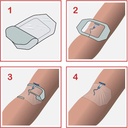
- Design of dressing allows aseptic application using a non-touch technique
- Split ensuring an efficient closure around the IV catheter
- Sterile, for single use
- Without anti-microbial agents
- Approximate size:
- small: 4 x 5 cm
- medium: 6 x 9 cm
- large: 10 x 11 cm
Packaging & Labelling
Each dressing is packed in a disposable peel pack allowing effective sterilization, safe handling, and storage until needed for use and facilitating proper aseptic presentation.
Instructions for use
Used to cover and protect catheter sites.
- Small: for pediatric use
- Large: use in critical contexts such as VHF or for Central venous catheters and PICC lines.
Precautions for Use
To ensure adequate dressing adhesion, the skin around the puncture site should be clean, dry and free of moisturizers and grease.
Caution is required when removing the dressing: stretch the film horizontally away from the wound to avoid damage to the skin
Storage
- Store below 25°C
- Protect from sunlight ‐ Protect from humidity
MSF requirements
The transparent dressing, easy to apply and remove, provides complete visibility of the site during application. It also allows continuous monitoring of the I.V. site without disturbing or removing the dressing.


![[KMEDMHIS22-] (mod ICU) SPECIFIC DRESSINGS complementary 2021](/web/image/product.template/574343/image_256/%5BKMEDMHIS22-%5D%20%28mod%20ICU%29%20SPECIFIC%20DRESSINGS%20complementary%202021?unique=4195f0f)