SKIN STAPLER, 35 regular staples , sterile, s.u.
Valid Article
SKIN STAPLER, + staples, sterile, s.u.
Definition
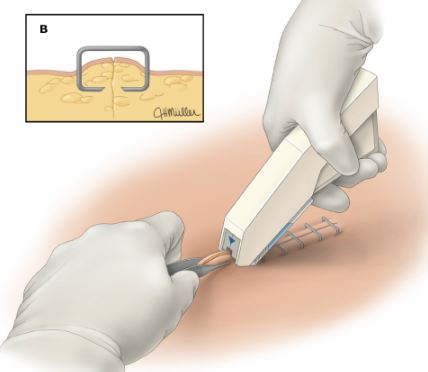
A sterile hand-held surgical instrument designed for the application of skin staples to approximate free edges of skin from an incision or wound. This device, often pistol-like in design, is typically loaded with staples and is intended to mechanically deliver staples with manual power. This is a single-use device.
Specifications
Technical specifications
STAPLER
- 35 rectangular staples are loaded in the stapler
- Indication of remaining staples
- Sterile, for single use
STAPLES
The construction of the staple is a design that folds up and ultimately makes a box shaped 'stitch' which holds the skin edges together but is slightly elevated from the natural level.
- Material: stainless steel (austenitic low carbon steel containing +/-3% molybdenum) or titanium
- Size: slight variations occur according to manufacturer
- Thickness: 0.5 – 0.6 mm
- Staple depth: 3.5 – 4.9 mm
- Backspan:
- Wide : 6.4 – 7.2 mm
- Regular : 5.0 – 5.9 mm
See picture
- 1 = original staple
- 2 = formed staple
- 3 = range of forming
Packaging & Labelling
Unit sterile packaging in peel open tray pack
Instructions for use
- Hold the skin edges together evenly by using a forceps.
- Place the centre mark of the stapler at the middle of the two skin edges.
- Squeeze the handle completely. Incompleteness of squeezing handle may cause incomplete forming of staple or improper stapling. Repeat above steps, while maintain same distance between each placed staples until the incision is closed. Any malformed staples should be removed and replaced with a new staple.
Precautions for Use
Make sure to press the handle COMPLETELY on the stapler in order to release the staple, otherwise the staple is not completely liberated and may block the next ones.
Storage
Protect from sunlight ‐ Protect from humidity