(mb GeneXpert) VAGINAL & ENDOCERVICAL KIT CT/NG SWAB/A-50
NST
ELAEMBIC116
Valid Article
HS Code:
901890
Last Updated on:
15/08/2025, 22:01:45
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
(mb GeneXpert) CT/NG VAGINAL & ENDOCERVICAL KIT SWAB-A50
Definition
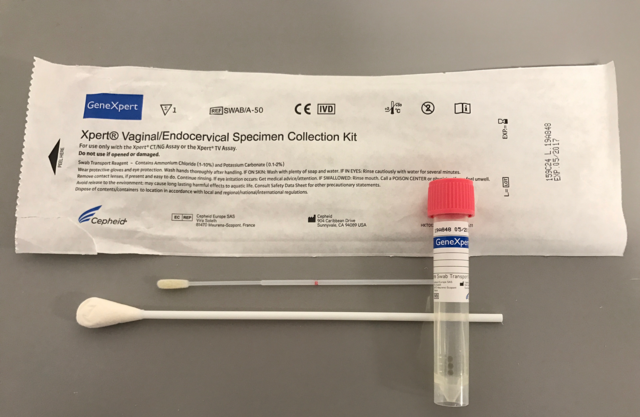
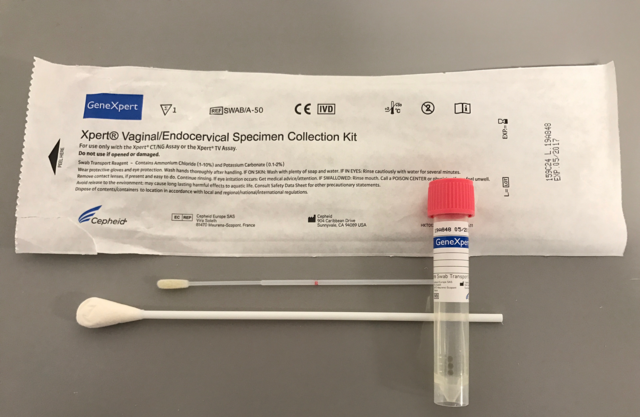
Swab with tube to collect, preserve and transport endocervical and vaginal specimens prior to analysis with the Xpert cartridges
Specifications
Some restricted information has been hidden. Sign in
to see this information
Components
50 kits per box containing:
- 1 large sterile cleaning swab, individuallly wrapped
- 1 flocked collection swab, individualllywrapped
- 1 tube swab transport reagent (pink cap
- )Instruction for collection of specimen (patient)-collected vaginal swab (50 per box
- )Instruction for collection of endocervical swabs and clinicial collected (1 per box)
Technical specifications
- Transportation temperature: 2-30°C
- Transport reagent is a clear, colorless and odorless liquid
Packaging & Labelling
50 kits/ pack
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Storage
- To be stored at 2 to 30 °C
- Shelflife up to 36 months
Classification EC Regulation N° 1272/2008
Not classified as hazardous
MSF requirements
Used for testing for Chlamydia trachomatis and Neisseria gonorrhoeae using the Xpert CT/GN cartridge, and Trichomonas vaginalis using the on TV Xpert cartridge.
Some restricted information has been hidden. Sign in
to see this information
Validated by Dominique Beels, laboratory advisor. July 2025