AUTOCLAVE 85 l (Tuttnauer 3870 HSG-WS-D), hospital, 230V
Valid Article
AUTOCLAVE 85l, hospital grade, class B, Tuttnauer HSG
Definition
This device is an AC-powered steam sterilizer (autoclave) designed to eliminate or inactivate microorganisms from medical devices and related materials using moist heat. It is intended for use with items not sensitive to high temperatures, water, or steam. The autoclave has an 85-litre chamber for placing devices or instruments and includes standard safety and control features. It can be used for both wrapped and unwrapped medical instruments.
The 3870 HSG is classified as a hospital-grade Class B autoclave. It is suitable for health facilities that require sterilization of complex hollow instruments, including devices with narrow lumens, such as internal fixators. It can run continuous sterilization cycles.
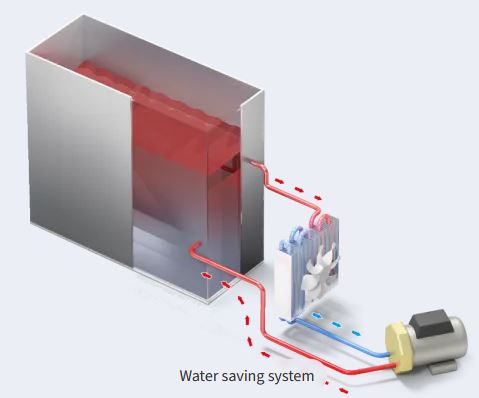
The HSG-WS variant incorporates a water-saving feature that recycles water within the cycle, reducing daily water consumption to approximately 5 litres. This supports more sustainable and efficient resource use in all settings.
Specifications
Pre & Post vacuum performance is achieved with a built-in vacuum pump used for fractionated pre vacuum air removal eliminating air pockets from all load types and maximizing efficient steam penetration throughout the entire load. After the sterilization stage the vacuum pump is used for post-vacuum drying.
Utility Connection Features
- Dependent on Utilities: the HSG can be operated as a stationary sterilizer connected to building utilities for distilled water, tap water, drain and electricity.
- Independent of Utilities: the HSG-WS comes with a standard “Plug and Play” option to operate only with an electric power connection. In this mode there is no need to connect the autoclave to the building utilities for distilled water, tap water and drain. With this option it is also possible to connect to utilities.
Quality standards
Technical specifications
- Double locking safety device prevents door from opening while chamber is pressurized
- Control lock-out switch prevents starting a cycle if door is not properly locked
- Control system protection prevents door from opening at high pressure and temperature
- water saving system - up to 5 liters per day only
- sleep mode - control system switches off when not in use
- delayed start timer - for after hours sterilization cycles (during low energy demand)
- fits through standard doorways
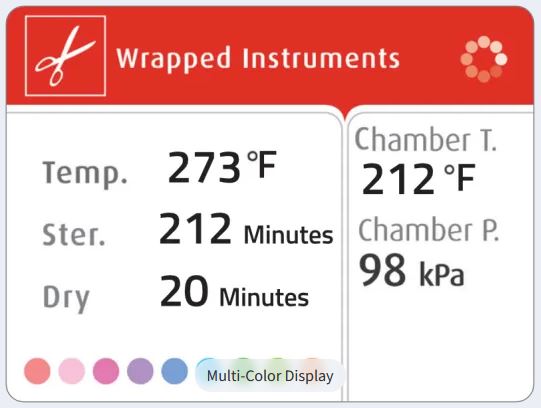
- multi-colored display with 3 button pad and alpha-numeric printer
- (4) FDA cleared cycles: unwrapped instruments, wrapped instruments, unwrapped delicate instruments, wrapped delicate instruments.
- Control Cycles: Self-Test, Leak Test, Vacuum Test, Bowie DIck Test
- Chamber dimensions : 384 x 758 mm
- Chamber volume : 85 litres
- Tray dimensions W x H x D : small : 286 x 25 x 675 mm, large : 350 x 25 x 675 mm
- Power: 3-Phase, 230Volts, 25 Amp., 60 Hz 9kW
Dimensions
- Overall dimensions W x H x D: 720 x 1365 x 1180 mm
- Autoclave weight: 180 kg
Instructions for use
which recommendations?
Precautions for Use
The project must consider access to quality water (either using a distiller or Reverse Osmosis plant). Contact your biomedical or water/sanitation referent for more information.
MSF requirements
This autoclave is essential for reprocessing complex hollow-body instruments with narrow lumens—such as internal fixators— ensuring effective steam sterilization.
Routinely monitor the sterilization procedure using mechanical, chemical, and biological indicators (BIs). This combination evaluates sterilizing conditions and the microbiologic status of processed items.
Quality Assurance tests required for the Tuttnauer Class B autoclave include:
- ESTEBIRE02- BIOLOGICAL INDICATOR READER / INCUBATOR (MiniBio)
- ESTEBIRT0201 – BIOLOGICAL INDICATOR, steam, air-removal, BT224
- ESTETBOD1-- BOWIE-DICK test pack, ready to use
- ESTETSTI51- TST INDICATOR STRIP, Type 5







![[ESTEAUTA8501] (aut. Tuttnauer 3870HSGWSD) CARRIAGE + LOADING CART](/web/image/product.template/587817/image_256/%5BESTEAUTA8501%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20CARRIAGE%20%2B%20LOADING%20CART?unique=749625a)
![[ESTEAUTC8501] (aut. Tuttnauer 3870HSGWSD) PAPER, roll, THE002-0066](/web/image/product.template/587823/image_256/%5BESTEAUTC8501%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20PAPER%2C%20roll%2C%20THE002-0066?unique=749625a)
![[ESTEAUTS8501] (autoclave Tuttnauer 3870M) DOOR SWITCH SRV000-0512](/web/image/product.template/587818/image_256/%5BESTEAUTS8501%5D%20%28autoclave%20Tuttnauer%203870M%29%20DOOR%20SWITCH%20SRV000-0512?unique=5b61cac)
![[ESTEAUTS8502] (aut. Tuttnauer 3870HSGWSD) DOOR GASKET, GAS080-0317](/web/image/product.template/587819/image_256/%5BESTEAUTS8502%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20DOOR%20GASKET%2C%20GAS080-0317?unique=749625a)
![[ESTEAUTS8504] (aut. Tuttnauer 3870) FILTER air 0.2mµ, FIL175-0066](/web/image/product.template/587820/image_256/%5BESTEAUTS8504%5D%20%28aut.%20Tuttnauer%203870%29%20FILTER%20air%200.2m%C2%B5%2C%20FIL175-0066?unique=749625a)
![[ESTEAUTS8505] (aut. Tuttnauer 3870HSGWSD) VALVE PLUNGER, SOL026-0029](/web/image/product.template/587821/image_256/%5BESTEAUTS8505%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20VALVE%20PLUNGER%2C%20SOL026-0029?unique=749625a)
![[ESTEAUTS8506] (aut. Tuttnauer 3870HSGWSD) SOLENOID VALVE, SOL026-0034](/web/image/product.template/587822/image_256/%5BESTEAUTS8506%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20SOLENOID%20VALVE%2C%20SOL026-0034?unique=749625a)
![[ESTEAUTS8507] (Tuttnauer 3870HSGWSD/2840EL-D) GASKET strainer, GAS082-0008](/web/image/product.template/579794/image_256/%5BESTEAUTS8507%5D%20%28Tuttnauer%203870HSGWSD-2840EL-D%29%20GASKET%20strainer%2C%20GAS082-0008?unique=3aa7369)
![[ESTEAUTS8508] (aut. Tuttnauer 3870HSGWSD) STRAINER FILTER, FIL175-0046](/web/image/product.template/587824/image_256/%5BESTEAUTS8508%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20STRAINER%20FILTER%2C%20FIL175-0046?unique=749625a)
![[ESTEAUTS8509] (aut. Tuttnauer 3870HSGWSD) GLASS steam gen., GLA028-0011](/web/image/product.template/587825/image_256/%5BESTEAUTS8509%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20GLASS%20steam%20gen.%2C%20GLA028-0011?unique=da85731)
![[ESTEAUTS8510] (aut. Tuttnauer 3870HSGWSD) MANUAL VALVE, VLV170-0035](/web/image/product.template/587826/image_256/%5BESTEAUTS8510%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20MANUAL%20VALVE%2C%20VLV170-0035?unique=da85731)
![[ESTEAUTS85100] (Tuttnauer 3870HSGWSD) CHECK VALVE spring d.1/4" ARM172-0007](/web/image/product.template/587866/image_256/%5BESTEAUTS85100%5D%20%28Tuttnauer%203870HSGWSD%29%20CHECK%20VALVE%20spring%20d.1-4%22%20ARM172-0007?unique=9321810)
![[ESTEAUTS85101] (Tuttnauer 3870HSGWSD) STRAIGHT MALE O-RING FIT100-0807](/web/image/product.template/587867/image_256/%5BESTEAUTS85101%5D%20%28Tuttnauer%203870HSGWSD%29%20STRAIGHT%20MALE%20O-RING%20FIT100-0807?unique=9321810)
![[ESTEAUTS85103] (Tuttnauer 3870HSGWSD) HEAT EXCHANGER ARM100-0152](/web/image/product.template/587869/image_256/%5BESTEAUTS85103%5D%20%28Tuttnauer%203870HSGWSD%29%20HEAT%20EXCHANGER%20ARM100-0152?unique=9321810)
![[ESTEAUTS85104] (aut. Tutt 3870HSGWSD) VALVE PTFE Solenoid 24VAC SOL026-0110](/web/image/product.template/587870/image_256/%5BESTEAUTS85104%5D%20%28aut.%20Tutt%203870HSGWSD%29%20VALVE%20PTFE%20Solenoid%2024VAC%20SOL026-0110?unique=9321810)
![[ESTEAUTS8513] (aut. Tuttnauer 3870HSGWSD) SWITCH pressure, THE005-0042](/web/image/product.template/587827/image_256/%5BESTEAUTS8513%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20SWITCH%20pressure%2C%20THE005-0042?unique=da85731)
![[ESTEAUTS8514] (aut. Tuttnauer 3870HSGWSD) SOLENOID 50Hz, SOL026-0116](/web/image/product.template/587828/image_256/%5BESTEAUTS8514%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20SOLENOID%2050Hz%2C%20SOL026-0116?unique=3aa7369)
![[ESTEAUTS8515] (aut. Tuttnauer 3870HSGWSD) SOLENOID ODE, SOL026-0036](/web/image/product.template/587829/image_256/%5BESTEAUTS8515%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20SOLENOID%20ODE%2C%20SOL026-0036?unique=da85731)
![[ESTEAUTS8516] (aut. Tuttnauer 3870HSGWSD) RELAY solenoid 24VDC,CTP201-0272](/web/image/product.template/587830/image_256/%5BESTEAUTS8516%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20RELAY%20solenoid%2024VDC%2CCTP201-0272?unique=3aa7369)
![[ESTEAUTS8517] (aut. Tuttnauer 3870HSGWSD) RELAY solenoid 24VAC,CTP201-0273](/web/image/product.template/587831/image_256/%5BESTEAUTS8517%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20RELAY%20solenoid%2024VAC%2CCTP201-0273?unique=da85731)
![[ESTEAUTS8520] (aut. Tuttnauer 3870) FUSE 1.25A, ELE035-0003](/web/image/product.template/587834/image_256/%5BESTEAUTS8520%5D%20%28aut.%20Tuttnauer%203870%29%20FUSE%201.25A%2C%20ELE035-0003?unique=d73ab4d)
![[ESTEAUTS8521] (aut. Tuttnauer 3870) FUSE 5x20mm 6.3A, SB, ELE035-0011](/web/image/product.template/587835/image_256/%5BESTEAUTS8521%5D%20%28aut.%20Tuttnauer%203870%29%20FUSE%205x20mm%206.3A%2C%20SB%2C%20ELE035-0011?unique=aa1cb33)
![[ESTEAUTS8523] (aut. Tuttnauer 3870) BOARD POWER SUPPLY ELE035-0216](/web/image/product.template/587836/image_256/%5BESTEAUTS8523%5D%20%28aut.%20Tuttnauer%203870%29%20BOARD%20POWER%20SUPPLY%20ELE035-0216?unique=aa1cb33)
![[ESTEAUTS8524] (aut. Tuttnauer 3870) SAFETY VALVE SVL029-0028-27](/web/image/product.template/587837/image_256/%5BESTEAUTS8524%5D%20%28aut.%20Tuttnauer%203870%29%20SAFETY%20VALVE%20SVL029-0028-27?unique=37ebf75)
![[ESTEAUTS8525] (aut. Tuttnauer 3870) WATER PUMP PUM055-0021](/web/image/product.template/587838/image_256/%5BESTEAUTS8525%5D%20%28aut.%20Tuttnauer%203870%29%20WATER%20PUMP%20PUM055-0021?unique=37ebf75)
![[ESTEAUTS8526] (aut. Tuttnauer 3870) WATER FILL ELECTRODE CMT196-0004](/web/image/product.template/587839/image_256/%5BESTEAUTS8526%5D%20%28aut.%20Tuttnauer%203870%29%20WATER%20FILL%20ELECTRODE%20CMT196-0004?unique=37ebf75)
![[ESTEAUTS8532] (aut. Tuttnauer 3870HSGWSD) FAN, 120x120x38, CTP201-0004](/web/image/product.template/587840/image_256/%5BESTEAUTS8532%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20FAN%2C%20120x120x38%2C%20CTP201-0004?unique=1d68630)
![[ESTEAUTS8533] (aut. Tuttnauer 3870HSGWSD) FAN, 150x172x38, CTP201-0159](/web/image/product.template/587841/image_256/%5BESTEAUTS8533%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20FAN%2C%20150x172x38%2C%20CTP201-0159?unique=1d68630)
![[ESTEAUTS8534] (aut. Tuttnauer 3870HSGWSD) HEATING ELEMENT HEA016-0049](/web/image/product.template/587842/image_256/%5BESTEAUTS8534%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20HEATING%20ELEMENT%20HEA016-0049?unique=1d68630)
![[ESTEAUTS8535] (aut. Tuttnauer 3870HSGWSD) GASKET GAS082-0112](/web/image/product.template/587843/image_256/%5BESTEAUTS8535%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20GASKET%20GAS082-0112?unique=1d68630)
![[ESTEAUTS8536] (aut. Tuttnauer 3870HSGWSD) ELECTRODE WATER LEV.SRV000-0502](/web/image/product.template/587844/image_256/%5BESTEAUTS8536%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20ELECTRODE%20WATER%20LEV.SRV000-0502?unique=d7271b7)
![[ESTEAUTS8537] (aut. Tuttnauer 3870HSGWSD) WATER PUMP PUM055-0031](/web/image/product.template/587845/image_256/%5BESTEAUTS8537%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20WATER%20PUMP%20PUM055-0031?unique=d7271b7)
![[ESTEAUTS8538] (aut. Tuttnauer 3870HSGWSD) CAPACITOR 12,5 μf, PUM055-0044](/web/image/product.template/587846/image_256/%5BESTEAUTS8538%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20CAPACITOR%2012%2C5%20%CE%BCf%2C%20PUM055-0044?unique=d7271b7)
![[ESTEAUTS8540] (aut. Tuttnauer 3870HSGWSD) THERMOSTAT THE005-0040](/web/image/product.template/587847/image_256/%5BESTEAUTS8540%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20THERMOSTAT%20THE005-0040?unique=d7271b7)
![[ESTEAUTS8541] (aut. Tuttnauer 3870HSGWSD) GAUGE pressure steam GAU029-0053](/web/image/product.template/587848/image_256/%5BESTEAUTS8541%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20GAUGE%20pressure%20steam%20GAU029-0053?unique=d7271b7)
![[ESTEAUTS8542] (Tuttnauer 3870HSGWSD/2840EL-D) SAFETY VALVE SVL029-0119](/web/image/product.template/579795/image_256/%5BESTEAUTS8542%5D%20%28Tuttnauer%203870HSGWSD-2840EL-D%29%20SAFETY%20VALVE%20SVL029-0119?unique=d7271b7)
![[ESTEAUTS8543] (aut. Tuttnauer 3870HSGWSD) SAFETY VALVE SVL029-0088](/web/image/product.template/587849/image_256/%5BESTEAUTS8543%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20SAFETY%20VALVE%20SVL029-0088?unique=d7271b7)
![[ESTEAUTS8544] (aut. Tuttnauer 3870HSGWSD) SWITCH pressure THE005-0021](/web/image/product.template/587850/image_256/%5BESTEAUTS8544%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20SWITCH%20pressure%20THE005-0021?unique=d7271b7)
![[ESTEAUTS8545] (aut. Tuttnauer 3870HSGWSD) SWITCH pressure THE005-0022](/web/image/product.template/587851/image_256/%5BESTEAUTS8545%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20SWITCH%20pressure%20THE005-0022?unique=d7271b7)
![[ESTEAUTS8546] (aut. Tuttnauer 3870HSGWSD) BUZZER, PIEZO, ELE035-0192](/web/image/product.template/587852/image_256/%5BESTEAUTS8546%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20BUZZER%2C%20PIEZO%2C%20ELE035-0192?unique=d7271b7)
![[ESTEAUTS8547] (aut. Tuttnauer 3870HSGWSD) SWITCH panel ELE035-0012](/web/image/product.template/587853/image_256/%5BESTEAUTS8547%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20SWITCH%20panel%20ELE035-0012?unique=a42795e)
![[ESTEAUTS8548] (aut. Tuttnauer 3870HSGWSD) VACUUM PUMP PUM057-0008](/web/image/product.template/587854/image_256/%5BESTEAUTS8548%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20VACUUM%20PUMP%20PUM057-0008?unique=a42795e)
![[ESTEAUTS8549] (aut. Tuttnauer 3870HSGWSD) GAUGE pressure GAU029-0050](/web/image/product.template/587855/image_256/%5BESTEAUTS8549%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20GAUGE%20pressure%20GAU029-0050?unique=a42795e)
![[ESTEAUTS8551] (aut. Tuttnauer 3870EA) CAPACITOR 470nF CTP201-0016](/web/image/product.template/587856/image_256/%5BESTEAUTS8551%5D%20%28aut.%20Tuttnauer%203870EA%29%20CAPACITOR%20470nF%20CTP201-0016?unique=05d0515)
![[ESTEAUTS8582] (aut. Tuttnauer 3870HSGWSD) STEAMTRAP 1/4" ARM100-0057](/web/image/product.template/587857/image_256/%5BESTEAUTS8582%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20STEAMTRAP%201-4%22%20ARM100-0057?unique=a42795e)
![[ESTEAUTS8583] (aut. Tuttnauer 3870HSGWSD) SWITCH, PRESSURE, THE005-0006](/web/image/product.template/587858/image_256/%5BESTEAUTS8583%5D%20%28aut.%20Tuttnauer%203870HSGWSD%29%20SWITCH%2C%20PRESSURE%2C%20THE005-0006?unique=a42795e)
![[ESTEAUTS8584] (aut. Tutt. 3870HSGWSD) SAFETY VALVE 2.8bar SVL029-0119.02](/web/image/product.template/587859/image_256/%5BESTEAUTS8584%5D%20%28aut.%20Tutt.%203870HSGWSD%29%20SAFETY%20VALVE%202.8bar%20SVL029-0119.02?unique=a42795e)
![[ESTEAUTS8585] (aut. Tutt. 3870HSGWSD) SAFETY VALVE 5.0bar SVL029-0088](/web/image/product.template/587860/image_256/%5BESTEAUTS8585%5D%20%28aut.%20Tutt.%203870HSGWSD%29%20SAFETY%20VALVE%205.0bar%20SVL029-0088?unique=a42795e)
![[ESTEAUTS8586] (Tuttnauer3870HSGWSD) TEMPERATURE SENSOR PT100x2 THE003-0004](/web/image/product.template/587861/image_256/%5BESTEAUTS8586%5D%20%28Tuttnauer3870HSGWSD%29%20TEMPERATURE%20SENSOR%20PT100x2%20THE003-0004?unique=a42795e)
![[ESTEAUTS8587] (Tuttnauer 3870HSGWSD) ELEC.LEVEL REGULATOR 230V CTP201-0198](/web/image/product.template/587862/image_256/%5BESTEAUTS8587%5D%20%28Tuttnauer%203870HSGWSD%29%20ELEC.LEVEL%20REGULATOR%20230V%20CTP201-0198?unique=a42795e)
![[ESTEAUTS8598] (Tuttnauer 3870HSGWSD) MICROSWITCH w/cable ELE036-0019](/web/image/product.template/587864/image_256/%5BESTEAUTS8598%5D%20%28Tuttnauer%203870HSGWSD%29%20MICROSWITCH%20w-cable%20ELE036-0019?unique=10ede19)
![[ESTEAUTS8599] (Tutt.3870HSGWSD) BRACKET,DOOR MICROSW.ACTIVATOR DOR387-0037](/web/image/product.template/587865/image_256/%5BESTEAUTS8599%5D%20%28Tutt.3870HSGWSD%29%20BRACKET%2CDOOR%20MICROSW.ACTIVATOR%20DOR387-0037?unique=9321810)
![[ESTEBIRE02-] BIOLOGICAL INDICATOR READER / INCUBATOR (MiniBio)](/web/image/product.template/587588/image_256/%5BESTEBIRE02-%5D%20BIOLOGICAL%20INDICATOR%20READER%20-%20INCUBATOR%20%28MiniBio%29?unique=8c38861)
![[ESTEBIRT0201] BIOLOGICAL INDICATOR, class B steam, BT224](/web/image/product.template/587587/image_256/%5BESTEBIRT0201%5D%20BIOLOGICAL%20INDICATOR%2C%20class%20B%20steam%2C%20BT224?unique=8c38861)