PICC, CH5, double lumen, catheter+ accessories, sterile,s.u.
STD
SINSPICC5D1
Valid Article
Account code:
60210
HS Code:
901839
Last Updated on:
30/06/2025, 01:00:53
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
C01020102 - Central i.v. multi-lumen catheters, peripheral access
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
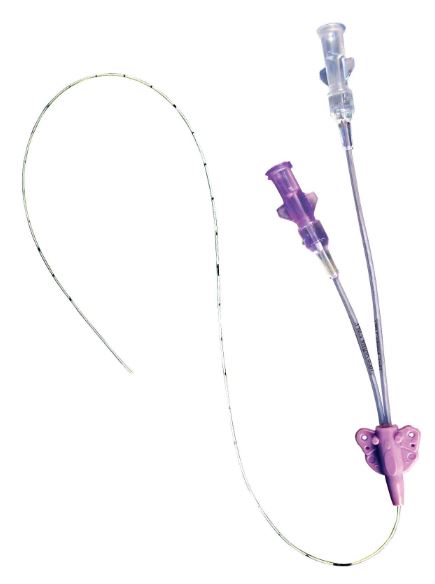
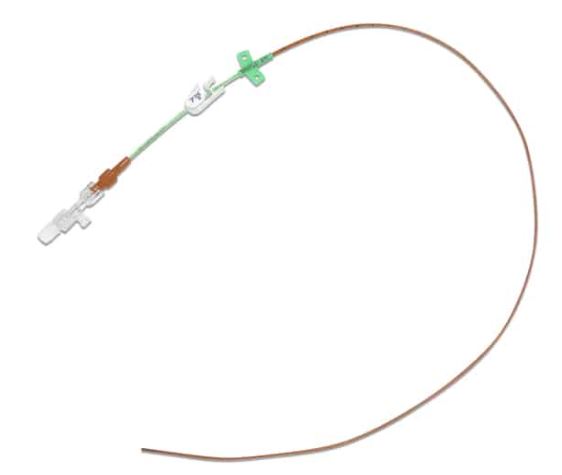
PERIPHERALLY-INSERTED CENTRAL CATHETER SET
Definition
A sterile, thin, flexible tube inserted into a peripheral vein and advanced to a large central vein for short- to long-term intravascular access. It allows for repeated access to the vascular system for up to 30 days or more for the administration of medications (antibiotics), chemotherapeutic agents, nutrients, parenteral solutions, pain management fluids, and may be used for blood sampling.
Synonym
PICC line
Specifications
Some restricted information has been hidden. Sign in
to see this information
Quality standards
- EN ISO 10555-1, 2013, edition 3, +A1 2017 Sterile, single-use intravascular catheters - Part 1: General requirements
- ISO 10555-3, 2013, edition 2, (confirmed 2018) Intravascular catheters - Sterile and single-use catheters - Part 3: Central venous catheters
Technical specifications
CATHETER
- Single-lumen or double lumen
- Length of catheter: varies according to manufacturer: 40 - 60 cm for CH3, 50 - 65 cm for CH5
- internal diameter:
- CH3 single lumen: 19G = 0.6 mm
- CH5 single lumen: 17G = 1.1 mm
- CH5 (or 4.5) double lumen: 2 x 18G
- Clearly labelled depth markings every 1 cm
- Soft tapered tip
- Open ended catheter with clamp (non valved)
- Composition of catheter: polyurethan (PUR)
- No antibiotic or antibacterial coating
- Sterile, for single use
ACCESSORIES for placement
- WIRE GUIDE
- length: 50 - 65 cm (varies according to manufacturer)
- diameter: 0.46 mm
- material: nitinol (or NiTi) is a metal alloy of nickel and titanium, where the two elements are present in roughly equal atomic percentages. Nitinol has special properties: shape memory effect and superelasticity, due to a martensitic phase transformation in the material.
- PUNCTION NEEDLE (percutaneous entry needle, introducer needle): Used for percutaneous entry in to vein using the seldinger technique
- 21G, 5 - 7 cm long
- clearly visible on ultrasound
- INTRODUCER: Luer-locking Peel-Away introducer sheath and dilator or metallic mandrin
- SYRINGE: 10-12 ml disposable Luer syringe
- SCALPEL: disposable, safety
- PAPER MEASURING TAPE
- CATHETER SECUREMENT DEVICE (see related article )
- NEEDLE-LESS CONNECTOR: one per lumen, split-septum needleless connector, neutral displacement (see related article)
- PROLONGATOR (Vygon): detachable, with Robert clamp
Sterile, for single use
Packaging & Labelling
Sterile, peel-open pack
To be Ordered Separately
The stabilization device and the needleless injection cap have to be changed at least weekly (see related articles).
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Precautions for Use
- The catheter tip can erode or perforate the vessel wall. Exercise utmost vigilance in the implementation and monitoring of PICC catheters.
- Positioning of the catheter tip should be routinely monitored by radiography
- In case the flow in the lumen is blocked, do not force the injection or evacuation of fluids.
Some restricted information has been hidden. Sign in
to see this information