MASK LARYNGEAL, reusable, size 2.5
Valid Article
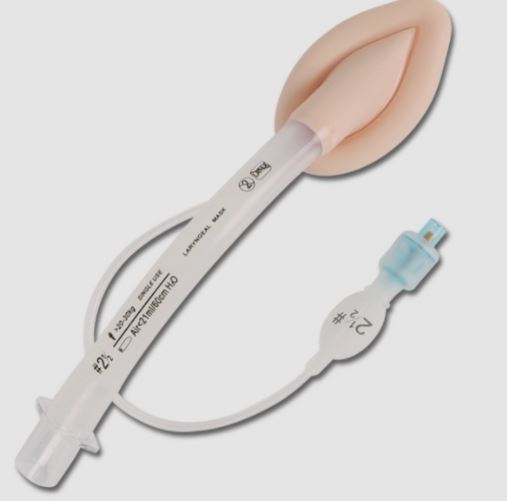
MASK LARYNGEAL
Definition
A curved tube used in inhalational anaesthesia and resuscitation to facilitate and secure airway patency for the delivery and exchange of gases in spontaneously breathing and ventilated patients.
It is inserted into the hypopharynx above the glottis to create a seal and to prevent the tongue from obstructing the anatomical airway. It may include a 15 mm connector that attaches to a breathing circuit or manual resuscitator, be radiopaque, and have a built-in pilot balloon for cuff pressure monitoring.
Synonym
LMA (Laryngeal Mask Airway)
Specifications
Quality standards
- ISO 11712, 2009, edition 1, Anaesthetic and respiratory equipment - Supralaryngeal airways and connectors
- ISO 18190, 2016, edition 1, Anaesthetic and respiratory equipment - General requirements for airways and related equipment
Technical specifications
Single piece moulded device, latex free
- Mask
- Ovoid, silicone, semi-rigid portion intended to be inserted into the pharynx
- inflatable peripheral cuff allowing to guarantee airtight seal over the glottic opening. Maximum pressure inside the cuff: 60 mm H2O
- Tube connected to the mask:
- translucent silicone
- curve that is similar to the one of a tracheal tube
- ended by a 15 mm connector allowing to fit on an Ambu® valve (and filter)
- Separate inflation line with valve and inflation pilot balloon allowing to inflate the cuff
- Autoclavable up to 134ºC
- Non sterile, reusable
- Sizes: see table below
Instructions for use
The laryngeal mask must always be used with an antiviral/antibacterial filter (see related articles below).
One unit of each size must be available in every structure having intubation equipment.
Precautions for Use
Some anaesthetic agents available within MSF, like ketamine, contraindicate this use.
Maintenance
Must be cleaned, pre-disinfected and sterilized by steam autoclave (deflated balloon) after each use.
(Cf Introduction: Disinfection and sterilization in the field)
Extra Tables
# 2 | # 2 1/2 | # 3 | # 4 | # 5 | |
Weight / poids patient | 10 – 20 kg | 20 – 30 kg | 30 – 50 kg | 50 – 70 kg | 70 – 100 kg |
Max volume LMA cuff / bourrelet | 10 ml | 14 ml | 20 ml | 30 ml | 40 ml |
Min. intern. Ø tube | 8.6 mm | 8.5 mm | 8.5 mm | 9.6 mm | 10.6 mm |
Max. extern. Ø tube | 15 mm | 17.5 mm | 17.5 mm | 20 mm | 22.5 mm |
Tube intern. length / longueur | 13.8 cm | 15.9 cm | 15.9 cm | 17.8 cm | 20.0 cm |
Intern. Ventilation volume | 11 ml | 15 ml | 16 ml | 21 ml | 30 ml |
Weight / poids | 24.6 g | 33.9 g | 38.3 g | 53.2 g | 73.0 g |