RECTAL MEDICATION APPLICATOR, plastic, s.u.
STD
SDDCREMAS1-
Valid Article
Account code:
60210
HS Code:
901839
Last Updated on:
08/08/2025, 22:01:39
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
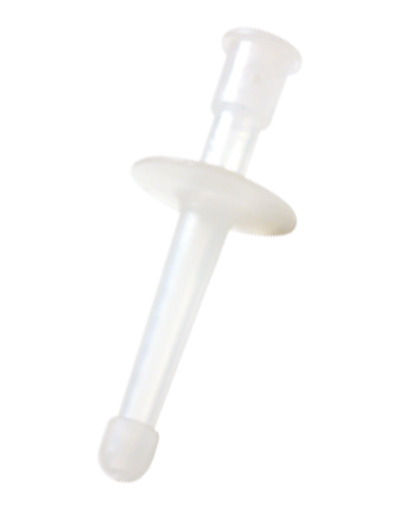
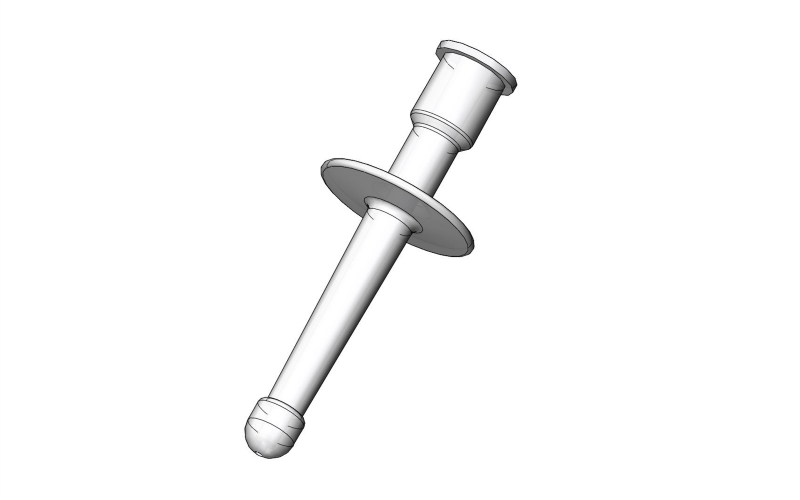
RECTAL MEDICATION APPLICATOR
Definition
Non-sterile device intended for rectal administration of medicines.
Plastic device in the form of a short tube to be connected to a syringe containing a medicine (diazepam solution).
Single-use device
Specifications
Technical specifications
- Hollow plastic tube with a ring stop
- Distal connection: for connection to a device containing the medication (e.g. syringe)
- Proximal end: olive-shaped
- Dimensions:
- total length: ± 53 mm
- insertion length (for rectal introduction): ± 33 mm
- proximal end diameter: 6 mm
- Without graduation
- Non-sterile
- Single-use
Packaging & Labelling
Box of 100 pieces
Instructions for use
In case of supply constraints with rectal cannula:
- administer midazolam intra-nasally using a mucosal atomization device as an alternative to rectal diazepam, or,
- administer diazepam intra-rectally using a syringe:
- Use a 1 ml syringe without needle
- Gently insert the syringe approximatively 1 cm into the anus, taking care not to cause harm to the child.
- After administration, firmly hold the child's buttocks together to prevent leakage of the medication.
Precautions for Use
Administer the medicine slowly.
Storage
Protect from sunlight - Protect from humidity
MSF requirements
Rectal cannula designed for the administration of diazepam solution by rectal route.
Some restricted information has been hidden. Sign in
to see this information


![[DINJDIAZ1A-] DIAZEPAM, 5mg/ml, 2ml, amp.](/web/image/product.template/571880/image_256/%5BDINJDIAZ1A-%5D%20DIAZEPAM%2C%205mg-ml%2C%202ml%2C%20amp.?unique=50acba7)