VENA CAVA CLAMP Satinsky, curved, 27cm 55-22-28
STD
ESURCLCSV27
Valid Article
Account code:
60100
HS Code:
903300
Last Updated on:
30/06/2025, 01:21:33
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
L0708010503 - Vena cava clamps, reusable
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
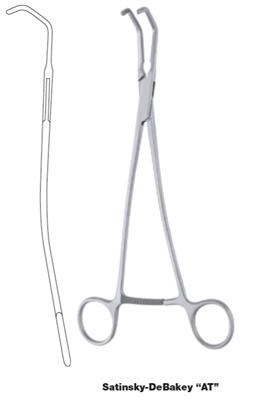
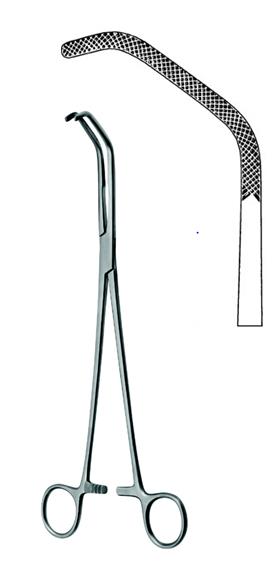
Vena cave clamp, Satinsky
Definition
Satinsky Vena Cava Clamp is a ratcheted, finger ring vascular clamp specifically designed for controlling blood flow in the vena cava vein. It is used in a variety of cardiovascular and cardiothoracic procedures. Long shanks and an angled, U-shape design allow this instrument to clamp difficult to reach sections of the vein.
This is a reusable surgical instrument intended to be sterilized prior to use.
Specifications
Technical specifications
- cross-serrated jaws, 45 mm long
- pediatric size, 27 cm total length
- progressive setting and locking of the ratchet
- angled
- material: martensitic steel
Some restricted information has been hidden. Sign in
to see this information


![[KSURBVAS5A-] VASCULAR SET, AORTA, 5 instruments + basket](/web/image/product.template/569422/image_256/%5BKSURBVAS5A-%5D%20VASCULAR%20%20SET%2C%20AORTA%2C%205%20instruments%20%2B%20basket?unique=1e7f2f5)