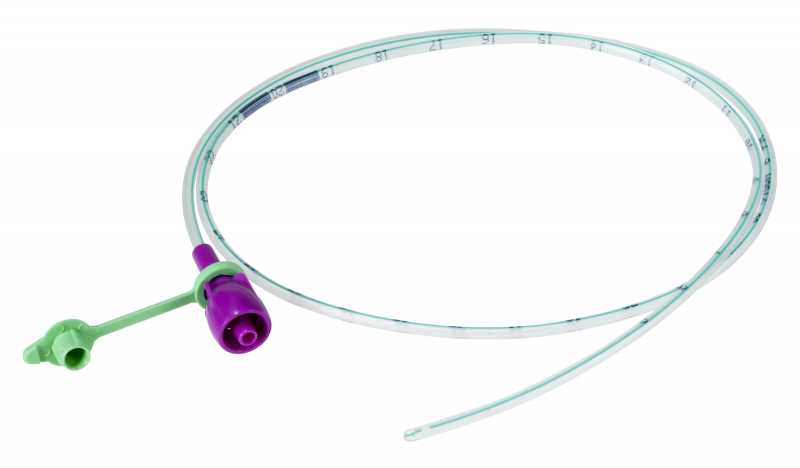
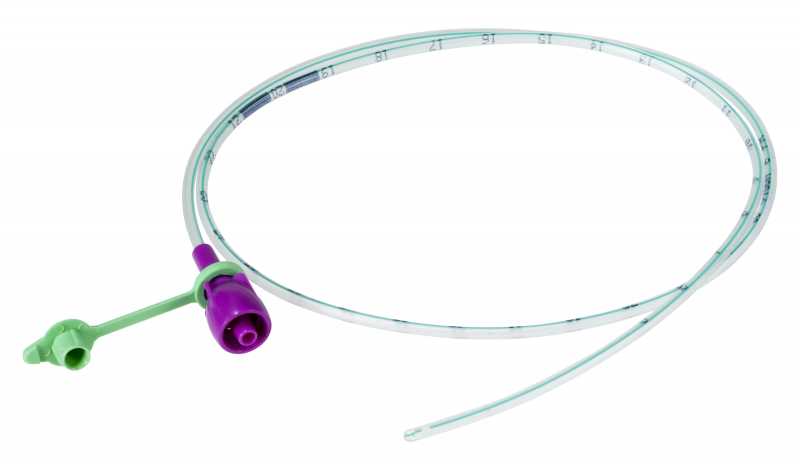
NASOGASTRIC TUBE, ENFit tip, s.u., 90 cm, CH12, white
Valid Article
NASOGASTRIC TUBE, ENFit tip
Definition
A sterile, thin, flexible, hollow cylinder intended to access the stomach of a patient through the nose and nasopharynx primarily for enteral feeding and the administration of medications, either by gravity or with a pump. It is typically a single-lumen plastic tube of various diameters (typically smaller than those of nasogastric tubes intended primarily for decompression) with circular markings that serve as insertion guides.
The NG-tube is designed with a specific ENFit end so that you can only use products designed for enteral/tube feeding access.
Specifications
Quality standards
Quality Standards Comment
See information on nasogastric and enteral tubes
Technical specifications
- Transparent
- PVC or polyurethane
- Single channel
- Closed distal end, with minimum 2 oval lateral eyes with atraumatic edges
- Proximal end with purple ENFit connection + stopper
- Radio-opaque or with radio-opaque markings for easy positioning (minimum / 5 cm)
- Possibility of identification of the size once the tube is out of the packaging, by colour code is preferred (coloured ring built into the connector, coloured closure cap, coloured attachement of the closure cap)
- Sterile, for single use
- Dimensions:
- Length: 40 to 60 cm (CH6 - 10) or 90 cm (CH12 & 14)
- Diameter: CH6, CH8, CH10, CH 12 and CH 14
- Size indicated by an international colour code:
| size | ext diam mm | colour |
| CH6 | 2.0 mm | light green |
| CH8 | 2.7 mm | blue |
| CH10 | 3.3 mm | black |
| CH12 | 4.0 mm | white |
| CH14 | 4.7 mm | green |
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Precautions for Use
- Confirm the correct position of nasogastric tubes
- Prevent misconnection errors
See information on nasogastric and enteral tubes



![[EEMDENPE13-] ENTERAL NUTRITION PUMP (Flocare Infinity-III) 230V + battery](/web/image/product.template/587339/image_256/%5BEEMDENPE13-%5D%20ENTERAL%20NUTRITION%20PUMP%20%28Flocare%20Infinity-III%29%20230V%20%2B%20battery?unique=b641e9b)