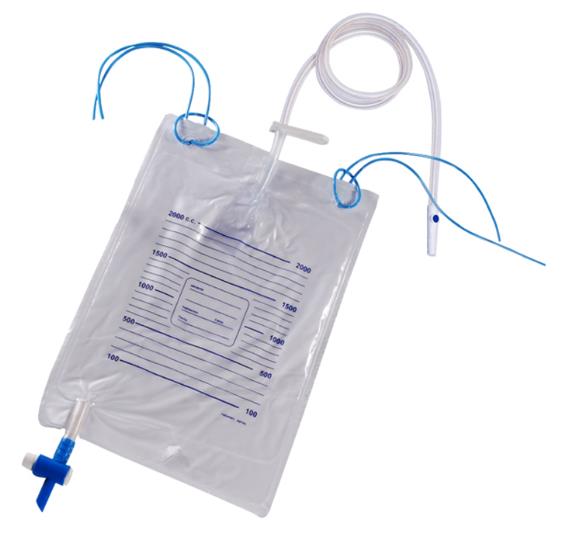
URINE BAG 2 l, drain., non-ret. valves, sampling port, ster.
Valid Article
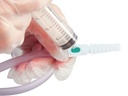
URINE COLLECTION BAG, with sampling port
Definition
A sterile flexible plastic pouch designed to connect to a urinary catheter to collect discharged urine from a patient. It is designed with an opening for urine drainage and a needle-free sampling port. The device is not directly attached to the patient.
Specifications
Quality standards
Technical specifications
- Bag:
- flexible, transparent plastic
- capacity: 2 litres
- graduations every 100 ml
- non-return valve at the tube inlet
- emptying system (T-shaped drainage tap)
- integrated hanging eyelets
- Drainage tube:
- flexible plastic
- length: ± 90 cm, diameter: 6 to 9 mm
- universal connector for connecting to a catheter, a urostomy pouch or a condom catheter/ incontinence sheath
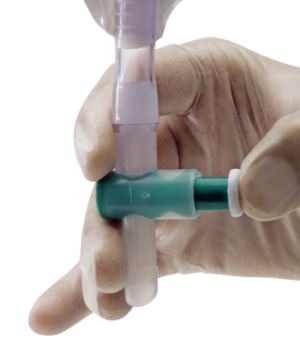
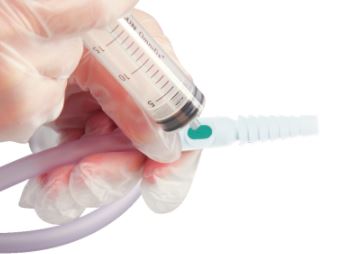
- needle-free sampling port
- protective cap
- Inside AND outside are sterile
- For single use
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Please consult the “Manual of nursing Care Procedures, MSF, 2020” available online via the Nursing care working Group sharepoint page.
Precautions for Use
A urine bag should never be left on the floor or hung onto a mobile structure which could cause strain such as pulling or tugging i.e. the bed rail. Provide hooks to attach the bag (see related articles below).
The drainage bag should always be positioned below the level of the patient's bladder and the healthcare provider should take care not to allow ‘back-flow' of urine into the bladder.
The drainage system should not be disconnected because this increases the risk of bacterial contamination. The catheter and the bag should be removed together.
It is important that samples are not taken from the bag (tap) because of multiplication of micro-organisms within this reservoir causing higher numbers. If no urine is visible in the tubing clamp it a few centimetres below the sampling port (do not clamp the catheter itself!). Wait until sufficient urine collects before proceeding with the sampling.






![[KMEDMHAS15-] (mod AMP) CATHETERS, TUBES & DRAINS](/web/image/product.template/572672/image_256/%5BKMEDMHAS15-%5D%20%28mod%20AMP%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS?unique=c0e1972)
![[KMEDMSUP04S1] (supplementary unit 2018) MODULE RENEWABLE SUPPLIES 2019](/web/image/product.template/569226/image_256/%5BKMEDMSUP04S1%5D%20%28supplementary%20unit%202018%29%20MODULE%20RENEWABLE%20SUPPLIES%202019?unique=c28d635)
![[KMEDMHIS25-] (mod ICU) CATHETERS, TUBES and DRAINS 2021](/web/image/product.template/574344/image_256/%5BKMEDMHIS25-%5D%20%28mod%20ICU%29%20CATHETERS%2C%20TUBES%20and%20DRAINS%202021?unique=4976048)
![[KMEDMHWS35-] (mod ward) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574358/image_256/%5BKMEDMHWS35-%5D%20%28mod%20ward%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=4d33748)
![[KMEDMHOS45-] (mod OT Room) CATHETERS AND DRAINS 2021](/web/image/product.template/574392/image_256/%5BKMEDMHOS45-%5D%20%28mod%20OT%20Room%29%20CATHETERS%20AND%20DRAINS%202021?unique=abfc26b)
![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=27e5983)
![[KMEDMHES35-] (mod emergency) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574355/image_256/%5BKMEDMHES35-%5D%20%28mod%20emergency%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=0590e60)
![[KMEDMSUP05S] (IEHK 2024 suppl. module) SUPPLEMENTARY RENEWABLE UNIT](/web/image/product.template/583185/image_256/%5BKMEDMSUP05S%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPLEMENTARY%20RENEWABLE%20UNIT?unique=9fce31f)
![[KMEDMHCS15-] (mod OPD) CATHETERS, TUBES & DRAINS](/web/image/product.template/572722/image_256/%5BKMEDMHCS15-%5D%20%28mod%20OPD%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS?unique=b7e0424)
![[SCTDBAGU2H-] (bag, urine) HOOK for FIXATION](/web/image/product.template/571900/image_256/%5BSCTDBAGU2H-%5D%20%28bag%2C%20urine%29%20HOOK%20for%20FIXATION?unique=2581d16)
![[SCTDBAGUF42] URINE FLOWMETER, grad. 400ml overflow 2l drain. bag, ster.](/web/image/product.template/571527/image_256/%5BSCTDBAGUF42%5D%20URINE%20FLOWMETER%2C%20grad.%20400ml%20overflow%202l%20drain.%20bag%2C%20ster.?unique=6c833ef)