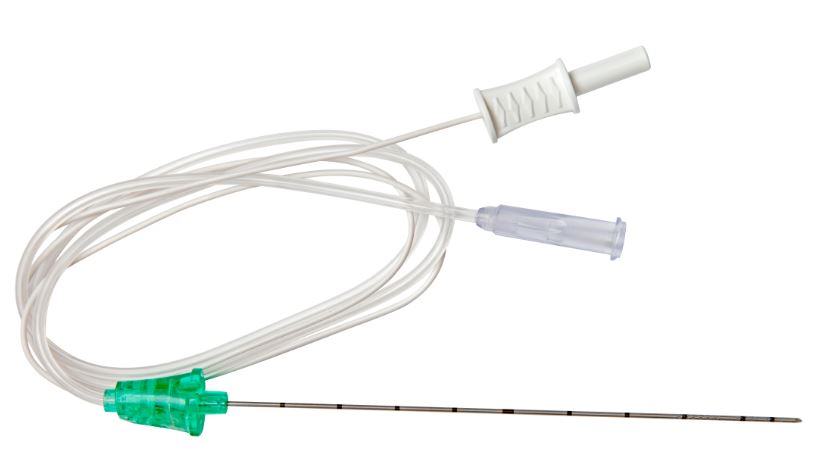
(Stimuplex HNS12) NEEDLE echogenic Luer 20Gx100mm 4892510-01
Valid Article
(nerve stimulator Stimuplex) NEEDLE echogenic
Definition
Hollow needle for electrical stimulation of peripheral nerves that facilitates the atraumatic performance of regional anaesthesia blocks.
The needles must be used with the nerve stimulator.
This technique allows the localisation of nerve plexuses and peripheral nerves with a motor component. During injection of local anaesthetics, the immediate simultaneous disappearance of the motor response helps to confirm the right position of the needle.
Synonym
stimulation cannula
Specifications
Quality standards
Technical specifications
- NEEDLE
- atraumatic 30° facet bevel
- insulated transparent coating on the whole length
- echogenic 360° X-pattern combined with a clear coating, reflects ultrasound waves back to the probe
- graduations every centimeter
- additional markings
- each 5 cm: 1 broad ring mark
- each 10 cm: 2 broad ring marks
- with protective cap
- INJECTION TUBE (= EXTENSION SET)
- transparent, 50 cm long, DEHP-free PVC
- distal end: female Luer Lock connector with stopper
- CONNECTING CABLE
- cable for connecting the nerve stimulator: Stimuplex HNS12
- Sterile, for single use
Comes in 3 sizes:
- 20 G = Ø 0.9 mm, length 100 mm
- 22 G = Ø 0.7 mm, length 50 mm
- 22 G = Ø 0.7 mm, length 35mm
Packaging & Labelling
Unit sterile packaging in peel-open pack. Box of 25.
Instructions for use
See user manual (Stimuplex HNS12)
Precautions for Use
To prevent accidental needlestick injuries, never recap the needle after use.
MSF requirements
The Stimulplex HNS12 needles should only be used with the selected nerve stimulator Stimuplex HNS 12.



![[EANENESE1--] PERIPHERAL NERVE STIMULATOR (Stimuplex HNS12), electrical](/web/image/product.template/569374/image_256/%5BEANENESE1--%5D%20PERIPHERAL%20NERVE%20STIMULATOR%20%28Stimuplex%20HNS12%29%2C%20electrical?unique=e897814)