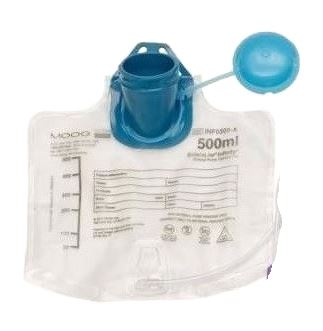
ENTERAL FEEDING RESERVOIR, top fill, 500 ml, + cap
Valid Article
ENTERAL FEEDING RESERVOIR
Definition
An empty pouch or bottle made of plastic intended to contain a fluid of nutrients for administration to a patient through an enteral tube that provides direct access to the stomach, duodenum, or jejunum. It is provided with a short length of tubing but is not intended to be directly connected to the feeding tube. This is a single-use device.
Specifications
Technical specifications
- Material : plastic transparent / translucent
- Easy to read graduations
- ENPlus connection to be connected to an administration set for enteral nutrition
- Large top fill opening with attached cap and hook or other means to attach the pouch to a pole.
- Volume: 500 ml or 1000 – 1600 ml
- Non sterile
- For single patient use
To be Ordered Separately
The administration set that connects the feeding bag with the nasogastric tube has to be ordered separately. see related articles.
Instructions for use
Precautions for Use
Single patient use. To avoid product contamination problems, the system (bag and administration set) should be replaced at least every 24 hours, or as needed.
- Therapeutic milk F75 must be used within 2 hours.
- ORS / Resomal should be used within 24 hours.
MSF requirements
The top-fill enteral nutrition bag allows the administration by gravity of non-ready to use solutions for enteral feeding (Therapeutic milk) or for enteral hydration (ORS or Resomal) if a close control of flow rate is required (critically ill malnourished patients) or when the volume to administer is too big to use 60 ml enteral syringe.


![[SCTDAENG1--] ADMINISTRATION SET EN, gravity, ENPlus/ENFit, ster.,s.u.](/web/image/product.template/571924/image_256/%5BSCTDAENG1--%5D%20ADMINISTRATION%20SET%20EN%2C%20gravity%2C%20ENPlus-ENFit%2C%20ster.%2Cs.u.?unique=6a4bbe0)
![[SCTDTUGF06-] NASOGASTRIC TUBE, ENFit tip, s.u., 50 cm, CH06, light green](/web/image/product.template/571563/image_256/%5BSCTDTUGF06-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2050%20cm%2C%20CH06%2C%20light%20green?unique=6a4bbe0)
![[SCTDTUGF08-] NASOGASTRIC TUBE, ENFit tip, s.u., 50 cm, CH08, blue](/web/image/product.template/571562/image_256/%5BSCTDTUGF08-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2050%20cm%2C%20CH08%2C%20blue?unique=6a4bbe0)
![[SCTDTUGF10-] NASOGASTRIC TUBE, ENFit tip, s.u., 50 cm, CH10, black](/web/image/product.template/571565/image_256/%5BSCTDTUGF10-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2050%20cm%2C%20CH10%2C%20black?unique=6a4bbe0)
![[SCTDTUGF12-] NASOGASTRIC TUBE, ENFit tip, s.u., 90 cm, CH12, white](/web/image/product.template/570057/image_256/%5BSCTDTUGF12-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2090%20cm%2C%20CH12%2C%20white?unique=43889b3)
![[SCTDTUGF14-] NASOGASTRIC TUBE, ENFit tip, s.u., 90 cm, CH14, green](/web/image/product.template/570060/image_256/%5BSCTDTUGF14-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2090%20cm%2C%20CH14%2C%20green?unique=43889b3)