CPAP O-Two single use SET, mask n°4 adult, + manometer
Valid Article
CPAP in line system o-two, with manometer
Definition
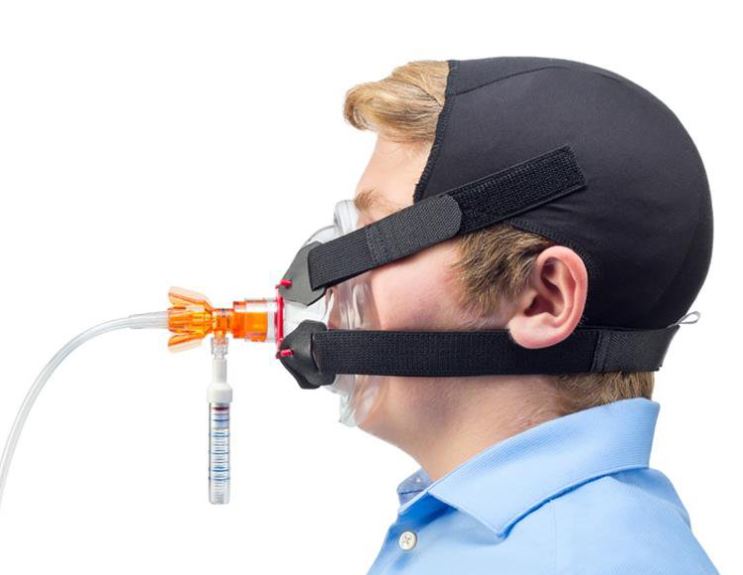
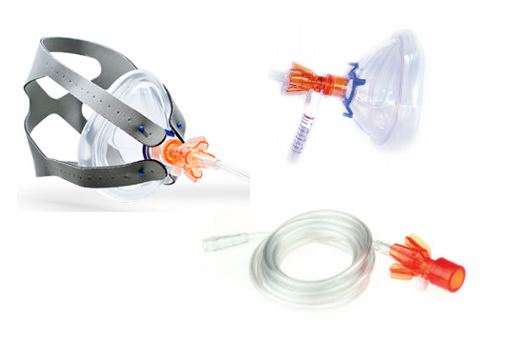
A system of devices intended to be integrated between a medical gas supply and a face mask/mouthpiece to assist noninvasive ventilation using continuous positive airway pressure (CPAP) during spontaneous patient respiration in respiratory emergency situations and/or respiratory therapy settings. It is a small plastic unit intended to be used with a separate flowmeter and manometer to establish a gas pressure proportional to gas flow rate through turbulent gas flow in an internal chamber using accelerating micro-channels (a virtual valve); tubing is included.
Specifications
The o-two Single-Use “Open CPAP” Delivery System provides accurate CPAP delivery.
A Luer port is located on the device to attach a pressure gauge. The ambient air intake port and the location of the in-line oxygen hose are designed to eliminate the possibility of accidental occlusion (risk of barotrauma).
The adjustment of the CPAP level is achieved by adjusting the output flow from your oxygen therapy regulator or wall outlet. The setting selections noted on the device provide an accurate constant airway pressure at each flow setting.
As this is an “Open” system, the device allows unrestricted inspiratory flows, since the patient has access to ambient air.
Technical specifications
- CPAP range: 5 – 25 cm H2O
- Pressure gauge range: 0 - 20 cm H2O
- Required therapy flow rate: 8 - 25 l / min
- Weight with face mask and tubing: 0.1 kg
- Input connection: oxygen therapy barb
- Patient face mask connection: 22mm
- Non sterile, single patient use
Packaging & Labelling
Each set is individually packed
Instructions for use
Precautions for Use
The system is not adapted for neonates and infants.
MSF requirements
Respiratory support in emergency for respiratory failure, alternative to invasive mechanical ventilation
Added value:
- light weight, portable
- ability to generate CPAP with oxygen flow of less than 10L (can work on oxygen concentrator)


![[KMEDMHIE21-] (mod ICU) EXAMINATION-RESUSCITATION EQUIPMENT](/web/image/product.template/574340/image_256/%5BKMEDMHIE21-%5D%20%28mod%20ICU%29%20EXAMINATION-RESUSCITATION%20EQUIPMENT?unique=cb394cc)
![[KMEDMHAE131] (mod AMP) ELECTRICAL MEDICAL EQUIPMENT](/web/image/product.template/568880/image_256/%5BKMEDMHAE131%5D%20%28mod%20AMP%29%20ELECTRICAL%20MEDICAL%20EQUIPMENT?unique=271efd5)
![[KMEDMHES35-] (mod emergency) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574355/image_256/%5BKMEDMHES35-%5D%20%28mod%20emergency%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=8014f55)