CD4 TEST, 200 cells/µl, wb, 1 test (Visitect OD376)
Outdated Article
CD4 TEST, 200 cells/µl (Visitect OD376)
This item is replaced by SSDTCD4T25T2, reason: new manufacturer and new reference. SSDTCD4T25T2 is not yet validated as MSF standard.
CAUTION
This test is worded and codified by unit to make the order easier, but it is always packaged in kit of 25.
Definition
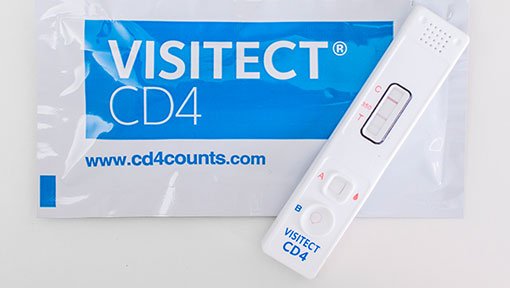
The VISITECT CD4 Advanced Disease Rapid Test is a manually operated semi-quantitative assay for the estimation of CD4 protein on the surface of CD4+ T cells in human whole blood (capillary or EDTA venous) to indicate whether the level is above or below 200 cells/μL within pre-diagnosed HIV patients.
This test is an aid in the management of patients with advanced HIV disease (patients with CD4 count below 200 cells/µl).
Specifications
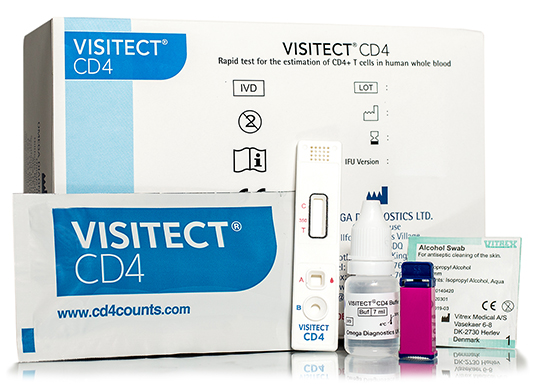
Components
- 5 foil pouch containing the test device and the desiccant
- 7mL x 1 bottle buffer
- 25 Sample devices
- 25 Retractable sterile lancets
- 25 Alcohol swabs
- 1 Job aid for whole blood specimens
- 1 Job aid for whole blood specimens
- 1 Instructions for use
Technical specifications
- Specimen: capillary or venous EDTA blood
- Time to result: 40-45 minutes
- Visual read
Packaging & Labelling
25 tests/pack
Instructions for use
Storage
- Store between 2-30°C. Do not freeze.
- Shelf life 12 months
- Guaranteed minimum remaining shelf life at delivery: 1/3 of total shelf life
MSF requirements
Follow-up of HIV patients in specific settings.
All orders of NST articles must be validated by your OC medical director.




![[ELAECD4E6--] CD4 ANALYZER (BD FACSPresto), 100-240V 50-60Hz + accessories](/web/image/product.template/570778/image_256/%5BELAECD4E6--%5D%20CD4%20ANALYZER%20%28BD%20FACSPresto%29%2C%20100-240V%2050-60Hz%20%2B%20accessories?unique=9514489)
![[SSDTCD4T25T2] CD4 TEST, 200 cells/µl, wb, 1 test (Visitect AB376)](/web/image/product.template/586623/image_256/%5BSSDTCD4T25T2%5D%20CD4%20TEST%2C%20200%20cells-%C2%B5l%2C%20wb%2C%201%20test%20%20%28Visitect%20AB376%29?unique=8e9161b)