OPHTALMIC/OTOLOGIC SET (Arclight)
Valid Article
OPHTALMIC/OTOLOGIC SET (Arclight SET)
Definition
The Arclight is a multi-purpose solar-powered diagnostic tool that combines a direct ophthalmoscope, anterior segment loupe and otoscope (as well as a number of other functions) into a small highly portable device
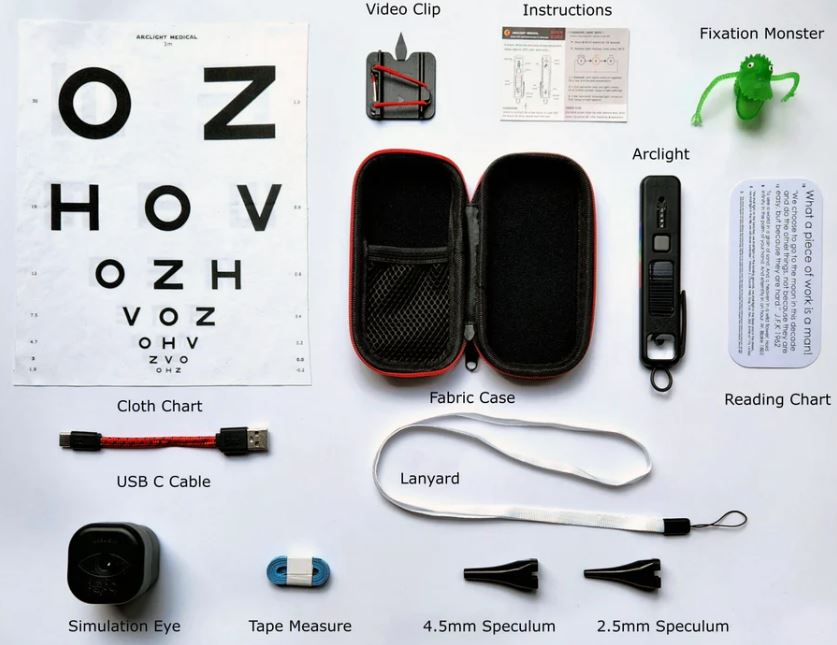
It comes in a case with a 3-metre visual acuity chart, clip to attach to a mobile phone camera to capture clinical signs, lanyard, speculae and a USB charging lead
Specifications
An all-in-one compact and easy-to-use ophthalmoscope-loupe-otoscope for healthcare professionals of all levels.LED – crisp and reliable illumination
- Blue light – for corneal exams
- Corrective lenses Anterior segment magnifying loupe
- Dual charge – solar panel & USB C
- Ear speculae convert to an otoscope
Technical specifications
- Dimensions & Weight:
- device – 110x24x9mm, 20g
- fabric case and extras – 127x67x33mm, 83g
- Materials: ABS, PC, PMMA, Silicone, Nylon
- Illumination: LED – warm-white (ophthalmoscope), daylight-white (otoscope/loupe), blue
- Logic Modes: 1) default general, 2) eye specialist, 3) ear specialist • Lenses: -3D, -6D, +4D
- Specula: 4.5mm and 2.5mm
- Battery & Charging: Li-Ion (enclosed). Solar and USB C
Supplied with the Article
Fabric Case:
- 2x Specula
- Lanyard
- USB C Cable
- Cloth Chart
- Reading Chart
- Video Clip
- Instructions/online content
Instructions for use
Recommended use in conjunction with tropicamide eye drops (DEXOTROP5D4) to dilate pupils
Precautions for Use
Proper study and training is essential.
Use clinical judgment: minimise light intensity and exposure.
Maintenance
Disinfect by wiping surfaces with alcohol. No user serviceable parts. Do not put in fluids or near flammable gases, use force, expose to high temperatures, or use if damaged.
MSF requirements
Description approved by referent of OCB (Tom Ellman) in February 2025.




![[DEXOTROP5D4] TROPICAMIDE, 0.5%, eye drops, sterile, unidose, amp.](/web/image/product.template/570795/image_256/%5BDEXOTROP5D4%5D%20TROPICAMIDE%2C%200.5%25%2C%20eye%20drops%2C%20sterile%2C%20unidose%2C%20amp.?unique=11d80ce)