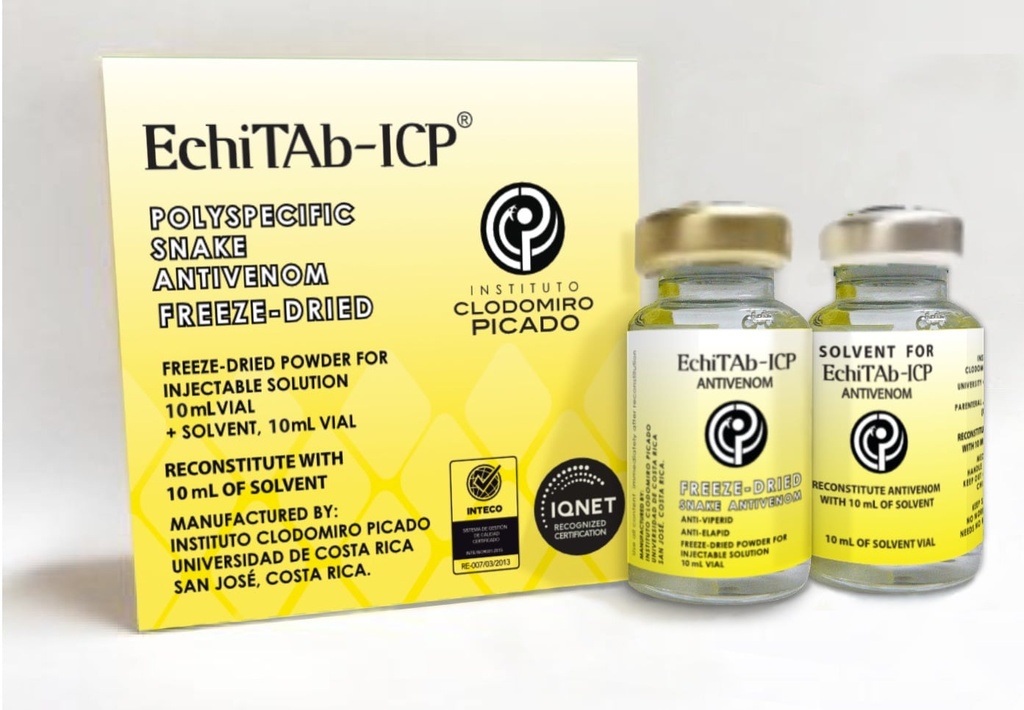
IMMUNOGL. SNAKE ANTIVENOM, Africa (EchiTab-ICP) pwd+dil vial
Valid Article
IMMUNOGLOBULIN SNAKE ANTIVENOM, Africa (EchiTab-ICP)
This product is not always available at supply centres level. Contact your HQ advisor and check the specifications if an alternative is offered.
Full Name
EchiTab-ICP, lyophilized Polyspecific Snake Antivenom
Therapeutic Action
Neutralisation of snake venom
Equine serum globulins
Indications
Treatment of snakebite envenomations from 22 African species found in sub-Saharan Africa, including:
- Puff adder and Gaboon vipers (Bitis spp.)
- African carpet viper (Echis spp.)
- African spitting cobra and neurotoxic cobras (Naja spp.)
- Black Mamba and green Mamba (Dendroaspis spp.)
Instructions for use
Lyophilised vial with diluent.
Reconstitute the powder vial with the diluent supplied to obtain a reconstituted 10 ml solution.
Must be given as:
- IV infusion in ringer lactate or 0.9% NaCl, or
- slow IV injection in case of vital emergency (fastest rate: 2ml/min)
The minimum dose for envenomation is 3 vials.
Read the manufacturer's instructions.
Precautions for Use
Never administer by IM route (ineffective).
Appropriate medical treatment should always be immediately accessible in the event of an anaphylactic reactions after the administration of the product.
Storage
- Below 25ºC
- After reconstitution, use the product immediately.

![[DVACIMAS3A-] IMMUNOGL. SNAKE ANTIVENOM, Africa (SAIMR), 10ml, amp.](/web/image/product.template/579010/image_256/%5BDVACIMAS3A-%5D%20IMMUNOGL.%20SNAKE%20ANTIVENOM%2C%20Africa%20%28SAIMR%29%2C%2010ml%2C%20amp.?unique=92612a4)