PLEURAL DRAINAGE SYSTEM, 1150ml, ster.s.u. (Sinapi XL1150SC)
Valid Article
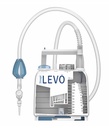
Sinapi Chest Drainage LEVO XL1150SC
Definition
The Sinapi Chest Drain is a sterile disposable drain device connected to a sterile catheter (not included) that is used to drain and store fluids away from the thoracic space.
Specifications
Chest Drain with Tubing, Dry Valve, Suction bulb, Suction Regulator, Tube roller, HNV, Single Collection Chamber 1150ml with Drainage Tap
sterile (EtO), for single use
shelf life = 5 years
Technical specifications
INLET TUBE:
- Flexible and able to milk - Suction bulb included
- Length: 135cm
- Translucent
- Contains a tube roller which facilitates tube stripping to prevent clot formation and maintains tube patency
- Contains a high negativity vent fitted in the connector, which counteracts the high negative pressures that are generated during stripping, making it a safe and efficient procedure
- Needle-free sampling port
- 3 connectors (S,M,L)
CONTAINER:
- 1150ml liquid holding capacity
- 1 chamber
- Includes outlet tap at bottom of holder to empty the holder
- Outlet tap fits onto Sinapi drainage container (D1150)
- Holder orientated upright or on its back possible without fluid spillage
- Integral flutter valve, ensuring flow of fluids away from the patient
- Integral air-leak detection chamber
- Low-pressure suction regulator with settings 0cmH2O to -40cmH2O pressure and feedback system indicating suction is applied - Suction dome to apply manual suction
Packaging & Labelling
packaging: single barrier system with protective packaging inside: blue sterilization wrap - Header bag = PE film bag with coated Tyvek strip
boxes of 18 units
Instructions for use
SET UP AND PREPARATION
Select the correct size female connector and attach it to the male end, rotating until a click is heard. Push the attached connector firmly into the chest catheter until it fits tightly. Secure the catheter and connecter with the provided cable tie. A luer-slip, needle free sample port is located on the connector from which samples may be taken.
No water is required for LEVO to function.
Ensure that it is placed lower than the patient in an upright position. Can safely be placed on its back on the bed (using the hangers located at the back of LEVO) for transfer or repositioning of the patient. No spillage or loss of seal will occur, provided that the drainage fluids do not exceed the MAX fill volume. LEVO can also be placed on the floor by rotating the stand found at the bottom of the collection chamber.
SUCTION
Mobile suction: As soon as the device is connected to the catheter, initiate mobile suction (and therefore drainage) by pressing the dome a few times. Suction is applied for as long as the dome is indented. Repeat the process to maintain suction.
Active suction: LEVO may be used on gravity drainage or active suction. Connect the tubing from the vacuum source to LEVO suction port. Turn the suction regulator to the prescribed value. Increase the active suction slowly until the red indicator ring is visible inside the suction regulator. Do not increase the active suction any further as LEVO will become noisy.
TUBE ROLLER AND SAFETY VENT
To milk the tubing, firmly pinch the tube roller onto the tubing and milk at least 50 cm of the tube length towards you in one smooth motion. The Safety Vent is fitted inside the connector and prevents excessively high negative pressures from being generated during tube milking. If done correctly, tube milking will induce a high pitched sound as the Safety Vent opens.
AIR LEAK DETECTION
Before checking for an air-leak, confirm that tube connections and catheter insertion dressing are air tight.
Step 1: Look for fluid above the Scheffler valve. Visible fluid indicates NO air-leak.
Step 2: Press the bulb. If it remains indented it indicates negative intrathoracic pressure and no air leak.
Step 3: If still in doubt, syringe saline/water into the back of the air leak chamber via the luer-lock, needle free port until the water level reaches the “fill to line”. Bubbling indicates an air leak. Continuous bubbling indicates a persistent air leak.
CONFIRMING CATHETER POSITION
Press the bulb; it should initially re-expand. If it stays indented, it indicates an absence of air at the catheter tip which may indicate that the catheter is NOT placed inside the pleural space. In the case of a hemothorax / pleural effusion, fluid should drain into the tubing as further confirmation of correct positioning
Precautions for Use
- This device is only intended for use by appropriately trained personnel.
- The connector contains the Safety Vent, if the connector is removed, the tube roller should not be used.
- Compatibility of LEVO with thoracic catheters needs to be established by the user.
- Regularly assess thoracic catheter connection for any signs of air leak.
- Neglecting removal of the Sinapi Vent Tool may cause lung collapse.
- Ensure that there are no dependent loops or kinks in the patient drainage tube.
- Little or no drainage may indicate a blocked thoracic catheter.
- Replace the LEVO if it is damaged or if there is evidence of occlusion.
- When using the air-leak chamber to monitor an air-leak, ensure that the water/saline level is maintained to the “fill to line” at all times.
- Do not keep the tube clamped during chest drainage, this will inhibit drain operation and may compromise respiratory function of patient. To temporarily clamp the tube, use the slot in the tube roller only. 1
- When using suction control, keep the device upright to prevent fluid from entering the suction regulator.
- Discontinue use and replace with a new unit if bodily fluids enter the suction regulator or air leak chamber.
- Observing Universal Precautions, discard LEVO in a medical waste container in accordance with the Waste Management policy of the health care facility.
NOTE: Any serious incident that occurs in relation to the device should be reported to the manufacturer. and the competent authority of the Member State in which the user and/or patient is established.
NOTE: Device volumes are approximate
Storage
storage temperature: < 25°C



![[SCTDPDSF1DS] PLEURAL DRAINAGE SYSTEM dry, 1X2L, ster. (Pleurevac S1100)](/web/image/product.template/580192/image_256/%5BSCTDPDSF1DS%5D%20PLEURAL%20DRAINAGE%20SYSTEM%20dry%2C%201X2L%2C%20ster.%20%28Pleurevac%20S1100%29?unique=a0b5785)