(clin. chem. i-STAT) CONTROL PTPlus lvl 2, kit, 5+5 06P17-18
Valid Article
(clinical chemistry i-STAT) KIT CONTROLS PT Plus
Definition
Two levels of control solution for PT and INR tests, to be used with the i-STAT PT Plus cartridge. Level 1 is normal/non-therapeutic and Level 2 is abnormal/therapeutic.
Specifications
Technical specifications
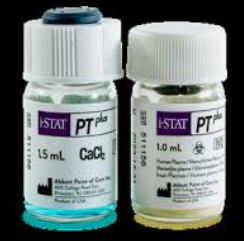
- i-STAT PT Plus controls, Levels 1 and 2 are composed of lyophilised plasma and CaCl2
- Lyophilised pellet, solid, straw yellow
- Solution liquid, blue
Dimensions
Box of 1 level control: 4.4 (H) x 11.7 (L) x 4.8 cm (P)
Packaging & Labelling
10 glass vials/box: Each level is composed of 5 vials of lyophilised human plasma + 5 vials of calcium chloride diluent
Transport Dangerous Goods
Transport not regulated
Instructions for use
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
Storage
- Keep refrigerated between 2 and 8ºC
- Do not freeze
- Shelf life: 12 months
- Guaranteed minimum remaining shelf life at delivery: 1/3 of the total shelf life
- Control solutions may also be stored at room temperature for up to 4 hours before reconstitution (18 to 30°C)
- Do not keep a reconstituted solution. It must be used immediately
Waste management
Dispose of this product as biohazardous waste according to all local, state, and
national regulations
Classification EC Regulation N° 1272/2008
Not classified as hazardous
Description validated by the OCB referent (Pascale Chaillet) in April 2025




![[ELAECCHE1--] CLINICAL CHEMISTRY ANALYSER (i-STAT)](/web/image/product.template/571409/image_256/%5BELAECCHE1--%5D%20CLINICAL%20CHEMISTRY%20ANALYSER%20%28i-STAT%29?unique=53b45fe)
![[ELAECCHT118] (clin. chem. i-STAT) CONTROL PT/INR lvl 2, kit, 5+5 06P17-14](/web/image/product.template/571308/image_256/%5BELAECCHT118%5D%20%28clin.%20chem.%20i-STAT%29%20CONTROL%20PT-INR%20lvl%202%2C%20kit%2C%205%2B5%2006P17-14?unique=f312659)
![[ELAECCHT138] (clinical chemistry i-STAT) CARTRIDGE PTPlus, 03P89-50](/web/image/product.template/587906/image_256/%5BELAECCHT138%5D%20%28clinical%20chemistry%20i-STAT%29%20CARTRIDGE%20PTPlus%2C%2003P89-50?unique=329bed2)