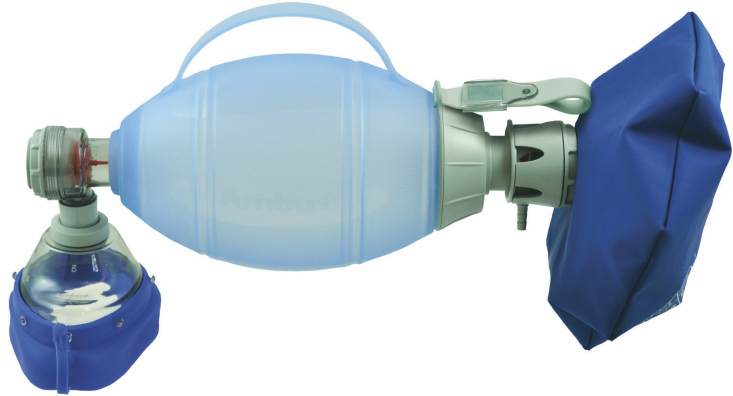
RESUSCITATOR MANUAL (Ambu), ad./child + masks RH5 / RH2
Valid Article
RESUSCITATOR adult/child (Ambu)
Definition
A hand-operated device designed to provide or assist ventilation in patients who are apnoeic or exhibit inadequate respiration. It employs entrained ambient air and includes a large flexible chamber that is hand-ventilated, a gas reservoir, tubing, and a connector for attachment to a mask or endotracheal tube; oxygen may also be connected when necessary.
It is used by emergency medical services in ambulances, intensive care units, during internal patient transfer, accident and emergency, mass casualty incidents, and is generally placed strategically throughout a hospital.
Specifications
Quality standards
Technical specifications
- SELF-INFLATING BAG
- self-inflating and compressible balloon in transparent silicone
- maximum bag volume: 1475 ml
- maximum stroke volume: 700 ml (can go up to 1100 ml if both hands are used)
- air intake valve
- patient valve connection
- fitted with a handle allowing the use with one hand only and regular inflations
- autoclavable 30x
- PATIENT VALVE
- non rebreathing device, one-way, adult model
- translucent polysulphone
- fitted with a pressure valve to avoid barotrauma, set at 40 cm H2O but can be blocked.
- with manometer port
- patient connector ISO 22/15 mm
- male respiratory connector ISO 30 mm, female respiratory connector ISO 24 mm
- dead space < 6 ml
- inspiratory resistance at 5 l/min: < or = to - 5 cm of H2O
- expiratory resistance at 5 l/min: < or = to 5 cm of H2O
- silicone red valve inlet
- removable expiratory outlet
- MASK nº RH2 / nº RH5
- transparent polysulphone shell
- silicone rubber blue rim
- fitted with metal hookring
- adjustable on 22/15 mm connections
- OXYGEN RESERVOIR BAG
- coated nylon (dark blue)
- capacity: 1500 ml
- autoclavable 15x
- Easy to disassemble for cleaning
- All components are autoclavable up to 134ºC
- Latex free
- Non sterile
Instructions for use
Forms part of the emergency resuscitation equipment. Should always be kept ready for use: visible, clean, complete and in good working order.
Should only be used by experienced staff.
The use of the oxygen reservoir bag is recommended if oxygen is available, as it enables to enrich the inspired gas with oxygen.
Precautions for Use
The patient valve is different for the adult/child self-inflating bag and the child self-inflating bag. The adult/child self-inflating bag must be used with the "adult" patient valve (see related articles below).
Maintenance
Before use:
- Check that the valve outlet operates correctly (it should not stick), by gently breathing through the valve whilst watching the flap move.
- Check the elasticity of the bag by manual pressure.
After each use:
- The patient valve and masks must be pre-disinfected, cleaned and sterilized by steam autoclave
(Cf Introduction: Disinfection and sterilization in the field)
MSF requirements
Device used for manual ventilation assistance in adults or children > 30 kg in case of respiratory distress or failure.






![[KMEDMHOE11-] (mod OT Room) RECOVERY EQUIPMENT, 2 beds](/web/image/product.template/572534/image_256/%5BKMEDMHOE11-%5D%20%28mod%20OT%20Room%29%20RECOVERY%20EQUIPMENT%2C%202%20beds?unique=2b32357)
![[KMEDMHHE22-] (mod hospital) BASIC RESUSCITATION EQUIPMENT](/web/image/product.template/572769/image_256/%5BKMEDMHHE22-%5D%20%28mod%20hospital%29%20BASIC%20RESUSCITATION%20EQUIPMENT?unique=80a635e)
![[KMEDMHOE17-] (mod OT Room) ANESTHESIA-RESUSCITATION EQ.](/web/image/product.template/572539/image_256/%5BKMEDMHOE17-%5D%20%28mod%20OT%20Room%29%20ANESTHESIA-RESUSCITATION%20EQ.?unique=ffed77c)
![[KMEDMHIE21-] (mod ICU) EXAMINATION-RESUSCITATION EQUIPMENT](/web/image/product.template/574340/image_256/%5BKMEDMHIE21-%5D%20%28mod%20ICU%29%20EXAMINATION-RESUSCITATION%20EQUIPMENT?unique=7433b39)
![[KMEDMHDE31-] (mod delivery & neonate) MEDICAL EQUIPMENT 2021](/web/image/product.template/574359/image_256/%5BKMEDMHDE31-%5D%20%28mod%20delivery%20%26%20neonate%29%20MEDICAL%20EQUIPMENT%202021?unique=010fcc3)
![[EANEANAA307] (resusc./ventilator) PEEP VALVE 20, inlet conn.30mm,reusable](/web/image/product.template/570697/image_256/%5BEANEANAA307%5D%20%28resusc.-ventilator%29%20PEEP%20VALVE%2020%2C%20inlet%20conn.30mm%2Creusable?unique=85683d8)
![[EANEMASARH2] MASK ANAESTHESIA + RIM + HOOK, size 2, child](/web/image/product.template/569434/image_256/%5BEANEMASARH2%5D%20MASK%20ANAESTHESIA%20%2B%20RIM%20%2B%20HOOK%2C%20size%202%2C%20child?unique=370142d)
![[EANEMASARH4] MASK ANAESTHESIA + RIM + HOOK, size 4, adolescent](/web/image/product.template/569421/image_256/%5BEANEMASARH4%5D%20MASK%20ANAESTHESIA%20%2B%20RIM%20%2B%20HOOK%2C%20size%204%2C%20adolescent?unique=6fbe0f6)
![[EANEMASARH5] MASK ANAESTHESIA + RIM + HOOK, size 5, adult](/web/image/product.template/569423/image_256/%5BEANEMASARH5%5D%20MASK%20ANAESTHESIA%20%2B%20RIM%20%2B%20HOOK%2C%20size%205%2C%20adult?unique=281bd43)
![[EANERESU201] (resuscitator Ambu adult/child) OXYGEN RESERVOIR complete](/web/image/product.template/569375/image_256/%5BEANERESU201%5D%20%28resuscitator%20Ambu%20adult-child%29%20OXYGEN%20RESERVOIR%20complete?unique=6fbe0f6)
![[EANERESU212] (manual resusc. Ambu) ADULT VALVE, pressure limit, complete](/web/image/product.template/580523/image_256/%5BEANERESU212%5D%20%28manual%20resusc.%20Ambu%29%20ADULT%20VALVE%2C%20pressure%20limit%2C%20complete?unique=32dd0c9)
![[EANERESU2CN] RESUSCITATOR MANUAL (Ambu), child + masks RH2/S1](/web/image/product.template/569376/image_256/%5BEANERESU2CN%5D%20RESUSCITATOR%20MANUAL%20%28Ambu%29%2C%20child%20%2B%20masks%20RH2-S1?unique=d0298c3)
![[SCTDBRCF1A-] FILTER, BREATHING CIRCUIT, 22M/15F, adult/child, s.u.](/web/image/product.template/571898/image_256/%5BSCTDBRCF1A-%5D%20FILTER%2C%20BREATHING%20CIRCUIT%2C%2022M-15F%2C%20%20adult-child%2C%20s.u.?unique=1495f79)