COUNTING CHAMBER, GLASSTIC, plastic, s.u., 1000 tests
STD
ELABCOCK1PD
Valid Article
Account code:
60200
HS Code:
901890
Last Updated on:
29/11/2025, 22:06:49
Former
Code(s):
ELAECOCK1PD
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
W0503900202 - Microscope slides coverslip
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
COUNTING CHAMBER GLASSTIC Kova
Definition
Slide designed for counting the cells in different biological fluids: blood, urine, CSF.
Specifications
Some restricted information has been hidden. Sign in
to see this information
Material
Plastic of high optic quality
Technical specifications
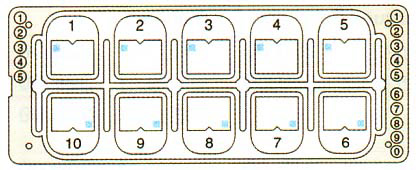
- Slide divided into 10 counting cells/wells (quantitative grids)
- Simple grid
- Each counting cell is an individual well including a grid with 9 big squares, each divided in 9 little squares
- Outer grid dimensions: 3 x 3 mm
- Chamber volume: 6.6 µl
- Chamber depth: 0.1 mm
- Grid volume = 1 counting cell: 0.9 µl
- Single use
Dimensions
- 12.2 cm x 9.1 cm x 7.6 cm
Packaging & Labelling
Box of 100 slides (= 1000 counting cells)
Instructions for use
Indicate the used grids in order not to re-use them.
Please consult the “Updated laboratory procedures, 2022” or “Bacteriology laboratory procedures and resources“ available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Home.aspx
For offline access, contact your laboratory advisor.
Waste management
To be destroyed by incineration.
MSF requirements
Easy to use in any context on different samples: urine, trypanosoma in CSF.
Some restricted information has been hidden. Sign in
to see this information





![[KMEDMHLA15-] (mod hospital lab) LABORATORY EQUIPMENT](/web/image/product.template/572580/image_256/%5BKMEDMHLA15-%5D%20%28mod%20hospital%20lab%29%20LABORATORY%20EQUIPMENT?unique=a241a8a)
![[KMEDMLAB16A] (laboratory module) BACT, HAEM, STOOLS, URINE EQUIP part A](/web/image/product.template/573182/image_256/%5BKMEDMLAB16A%5D%20%28laboratory%20module%29%20%20BACT%2C%20HAEM%2C%20STOOLS%2C%20URINE%20EQUIP%20part%20A?unique=3b1788a)