PIPETTE DISPENSER (Multipette M4 Eppendorf), manual adj.
STD
ELABPIAD5--
Valid Article
Account code:
60200
HS Code:
901890
Last Updated on:
01/12/2025, 22:03:24
Former
Code(s):
ELAEPIMA5--
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
W050302010102 - Serological and dilution pipettes, plastic
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
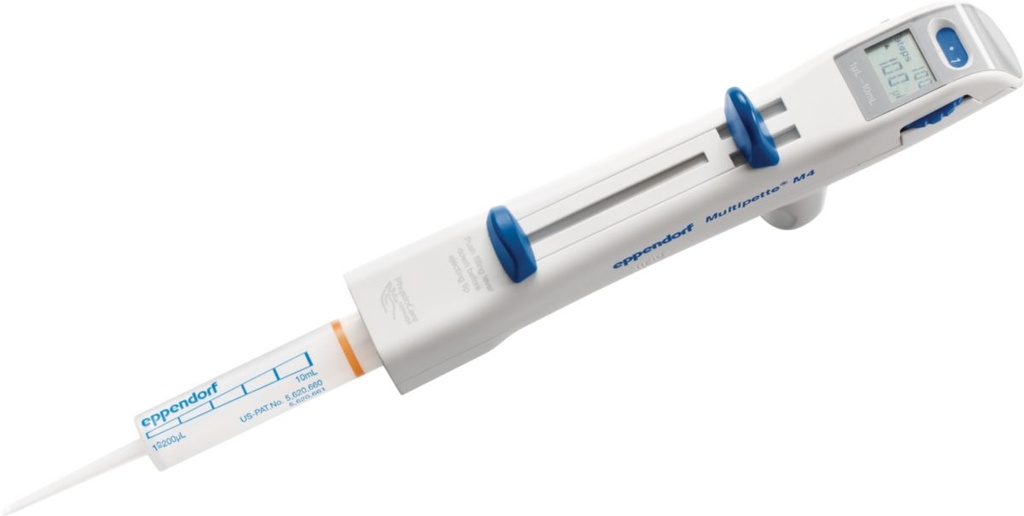
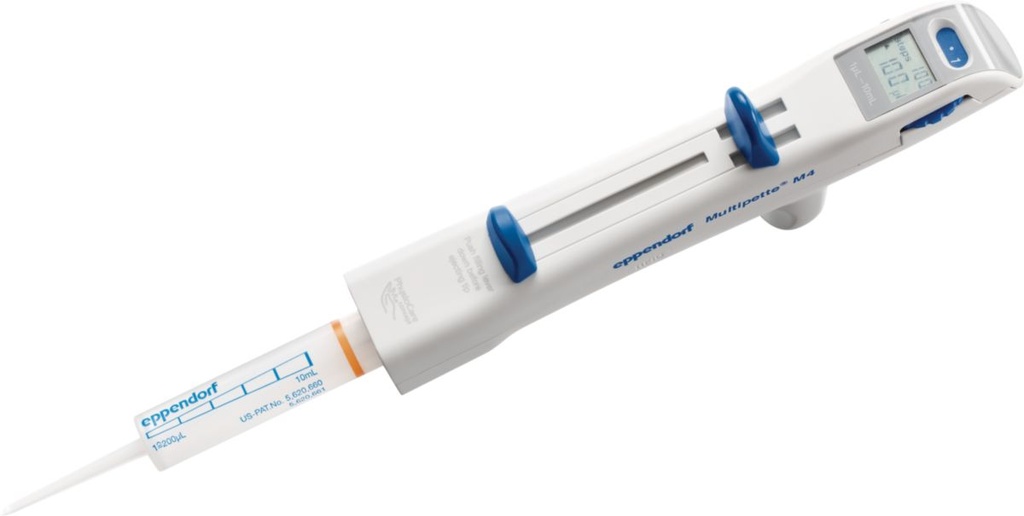
PIPETTE DISPENSER (Multipette M4)
Definition
Manual hand held dispenser for serial pipetting without refilling. It is adjustable with several tips that covers a wide range of volumes.
Specifications
Some restricted information has been hidden. Sign in
to see this information
Material
Polypropylene (PP)
Technical specifications
- 1 channel
- Volume range: 1µl–10ml (depends on the Combitip size)
- Volume adjuster lever: 5 positions
- Digital display
- Mechanical bolt for syringe
- Filling plunger
- Distribution lever
Packaging & Labelling
Unit packaging.
Instructions for use
To be used with syringes Combitips® advanced (to be ordered separately: see related articles below).
MSF requirements
Easy and rapid distribution for tests performed on microtitration plate.
Suitable volume for the needs of the leishmaniasis DAT test. See checklist Kala-Azar test (DAT).
Recommended in TB laboratories that process a large number of samples in MGIT liquid culture.
Some restricted information has been hidden. Sign in
to see this information
Some restricted information has been hidden. Sign in
to see this information

![[ELABPIAD51-] PIPETTE DISPENSER (Multipette M4 Eppendorf) + wall support](/web/image/product.template/574952/image_256/%5BELABPIAD51-%5D%20PIPETTE%20DISPENSER%20%28Multipette%20M4%20Eppendorf%29%20%2B%20wall%20support?unique=3f55097)
![[ELABPIADT100] TIP for pipette dispenser, 10ml (Combitips Eppendorf)](/web/image/product.template/568735/image_256/%5BELABPIADT100%5D%20TIP%20for%20pipette%20dispenser%2C%2010ml%20%28Combitips%20Eppendorf%29?unique=9db45b3)
![[ELABPIADT25] TIP for pipette dispenser, 2.5 ml (Combitips Eppendorf)](/web/image/product.template/570957/image_256/%5BELABPIADT25%5D%20TIP%20for%20pipette%20dispenser%2C%202.5%20ml%20%28Combitips%20Eppendorf%29?unique=5471cb6)