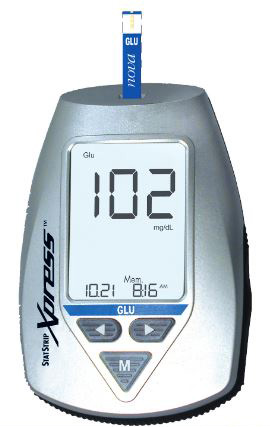
GLUCOMETER, blood glucose monitor (StatStrip Xpress) mg/dl
Valid Article
GLUCOMETER (Nova Statstrip Xpress)
CAUTION: The strips and control solutions, to be ordered separately, are specific to the glucometer type and brand.
Definition
Handheld glucometer for the quantitative determination of glucose concentration in whole blood.
Specifications
Blood glucose in whole blood from capillary collection
Quality standards
Technical specifications
- Measuring range: 10-600 mg/dl or 0.6-33.3 mmol/l
- Unit of measure: mg/dl or mmol/l
- Possible haematocrit range: 20-65 %
- Operating temperature: 15-40°C
- Operating relative humidity: 10-90 %
- Result display after 6 seconds
- Data storage: 400 results
- Battery-operated: coin cell Lithium Manganese dioxide battery 3V CR2450
Dimensions
- Size: +/- 150 x 82 x 46 mm
- Weight: 360 g
Transport Dangerous Goods
- UN3091: Lithium metal batteries packed with equipment
- Class: 9
- Packing instructions: 970 Section II
- Limit per package:
- Pax A/C (Passengers & Cargo Aircraft) = 5 kg
- CAO (Cargo Aircraft Only) = 5 kg
Supplied with the Article
Protection box (ref: 90023): protection against dust
To be Ordered Separately
- 200 strips: 2 boxes of 100 (ref: 42214)
- Control solutions: low (ref. 46947), normal (ref. 46948) and high (ref. 46949)
(see related items)
Instructions for use
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
Maintenance
Never repair. Replace with new one if broken.
MSF requirements
Handheld automatic analyzer allowing direct and quantitative determination of glycaemia.
Analyzer authorized for a shared use.
Accurate and reliable results also for paediatric use (because there is no interference with haematocrit).
Note
The glucometer StatStrip Xpress (ref. 47073 and 47074) allows the determination of both glycemia and ketonemia. Measurement of ketones requires separate strips. Ordering of these strips requires validation of your medical director (ref. 46951)

![[KMEDMHLA11-] (mod hospital lab) RAPID DIAGN. TESTS + GLUCOMETER +HEMOCUE](/web/image/product.template/572569/image_256/%5BKMEDMHLA11-%5D%20%28mod%20hospital%20lab%29%20RAPID%20DIAGN.%20TESTS%20%2B%20GLUCOMETER%20%2BHEMOCUE?unique=270b93f)
![[KMEDMSUP04D] (supplementary unit 2018) MODULE RTR](/web/image/product.template/572555/image_256/%5BKMEDMSUP04D%5D%20%28supplementary%20unit%202018%29%20MODULE%20RTR?unique=c3a8dd3)
![[KMEDMSUP05GD] (IEHK 2024 suppl. module) SUPPLEMENTARY GLUCOMETER RTR UNIT](/web/image/product.template/583183/image_256/%5BKMEDMSUP05GD%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPLEMENTARY%20GLUCOMETER%20RTR%20UNIT?unique=e07fede)
![[KMEDMNUT34A] (module nutrition) DIAGNOSTIC MATERIALS 2021](/web/image/product.template/575286/image_256/%5BKMEDMNUT34A%5D%20%28module%20nutrition%29%20DIAGNOSTIC%20MATERIALS%202021?unique=778b2b4)
![[ELAEGLUA202] (glucometer Nova StatStrip) STORAGE BOX](/web/image/product.template/572286/image_256/%5BELAEGLUA202%5D%20%28glucometer%20Nova%20StatStrip%29%20STORAGE%20BOX?unique=2a8435b)
![[ELAEGLUA203] (glucometer Nova StatStrip) BATTERY 3V, DL2450](/web/image/product.template/572285/image_256/%5BELAEGLUA203%5D%20%28glucometer%20Nova%20StatStrip%29%20BATTERY%203V%2C%20DL2450?unique=66685bf)
![[ELAEGLUT201] (glucometer Nova StatStrip) STRIP 42214](/web/image/product.template/571950/image_256/%5BELAEGLUT201%5D%20%28glucometer%20Nova%20StatStrip%29%20STRIP%2042214?unique=801ab68)
![[ELAEGLUT211] (glucometer Nova StatStrip) CONTROL SOL. low, 4ml vial 46947](/web/image/product.template/571951/image_256/%5BELAEGLUT211%5D%20%28glucometer%20Nova%20StatStrip%29%20CONTROL%20SOL.%20low%2C%204ml%20vial%2046947?unique=8e69c8c)
![[ELAEGLUT212] (glucometer Nova StatStrip) CONTROL SOL.normal 4ml vial46948](/web/image/product.template/571957/image_256/%5BELAEGLUT212%5D%20%28glucometer%20Nova%20StatStrip%29%20CONTROL%20SOL.normal%204ml%20vial46948?unique=568dbd6)
![[ELAEGLUT213] (glucometer Nova StatStrip) CONTROL SOL.high, 4ml vial 46949](/web/image/product.template/571959/image_256/%5BELAEGLUT213%5D%20%28glucometer%20Nova%20StatStrip%29%20CONTROL%20SOL.high%2C%204ml%20vial%2046949?unique=8e69c8c)
![[STSSLANCSAM2] SAFETY LANCET, medium flow, needle 21G x 1.8mm, green, s.u.](/web/image/product.template/569100/image_256/%5BSTSSLANCSAM2%5D%20SAFETY%20LANCET%2C%20medium%20flow%2C%20needle%2021G%20x%201.8mm%2C%20green%2C%20s.u.?unique=cba78fa)
![[STSSLANCSHL1] SAFETY LANCET, low flow, needle 28Gx1.6mm, light blue, s.u.](/web/image/product.template/570578/image_256/%5BSTSSLANCSHL1%5D%20SAFETY%20LANCET%2C%20low%20flow%2C%20needle%2028Gx1.6mm%2C%20light%20blue%2C%20s.u.?unique=a23035c)