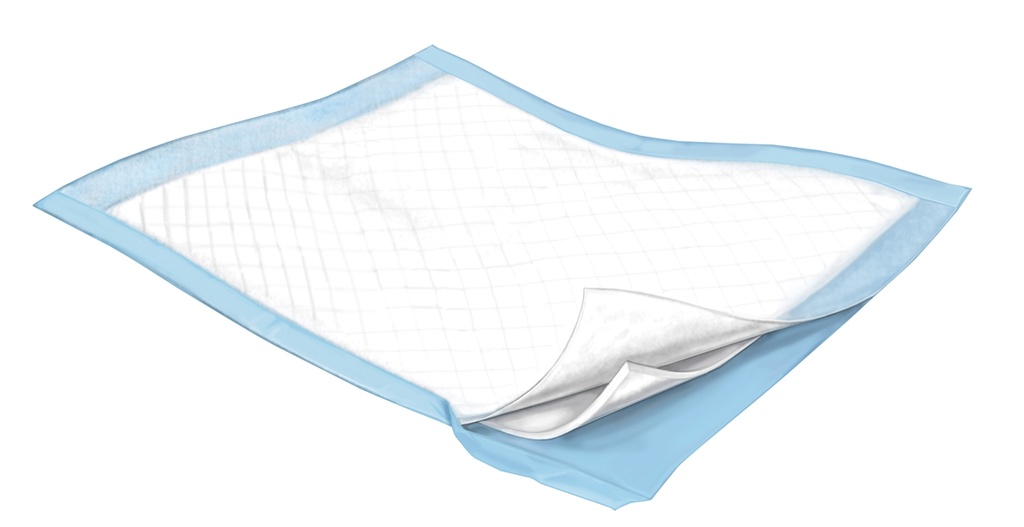
DRAWSHEET, disposable, plastified, absorbable, 60 x 60 cm
Valid Article
DRAWSHEET, disposable
Definition
A non-sterile padded sheet intended to cover and protect a piece of hospital furniture (bed, examination table) by catching and retaining liquids. It is typically a multi-layered piece of fabric containing a mixture of material types: synthetic (e.g., polypropylene fabric) and plant-derived materials (e.g., cellulose).
Synonym
Chux, bed pads, mattress pads, and chair pads
Specifications
Quality standards
Technical specifications
- Size: +/- 60 x 60 cm
- Non sterile, disposable
Different layers:
- Topsheet: For patient comfort it has a nonwoven material on the surface which should be kind to the skin
- Core: fluff +/- polymer provides moderate incontinence protection and odor control (virgin fluff pulp is the cellulosic part of the absorbent core of softwoods which contains coarse, bulky, long fibres which provide increased fluid retention and liquid distribution)
- Backsheet: waterproof plastic (polypropylene, latex-free) to protect against leakage which is sealed on 4 sides
- The liquid / discharge is dispersed quickly within the pad quickly enough to prevent spillage. For this purpose an additional diffuser veil may be placed between the core and the topsheet. The surface of the pad remains dry when moistened.
Absorption: moderate = total ISO absorbency 600 – 1000 ml (Rothwell scale 7-8) Underpads will be more or less absorbent depending on the amount of water-absorbing polymer or fluff, not on the size or thickness of the pad.
Packaging & Labelling
Depends on the brand by 28, 50, 100 etc.but never by piece
Instructions for use
Precautions for Use
it is not recommended to use an incontinence product to its full capacity, as it will be saturated, cause the product to be heavy, uncomfortable and damp against the skin with a risk of skin irritation.




![[PHYPISHE9020W] INCONTINENCE SHEET, ± 90x90cm, ± 2000 ml/m², washable](/web/image/product.template/576278/image_256/%5BPHYPISHE9020W%5D%20INCONTINENCE%20SHEET%2C%20%C2%B1%2090x90cm%2C%20%C2%B1%202000%20ml-m%C2%B2%2C%20washable?unique=900fbdc)
![[KMEDMEBO12A] (module VHF) MODULE SUPPORT 1 HOSPITAL + 10 PHC](/web/image/product.template/571737/image_256/%5BKMEDMEBO12A%5D%20%28module%20VHF%29%20MODULE%20SUPPORT%201%20HOSPITAL%20%2B%2010%20PHC?unique=9ba2a24)
![[KMEDMEBO13A] (module VHF) MODULE ENVIRONMENTAL HEALTH](/web/image/product.template/571761/image_256/%5BKMEDMEBO13A%5D%20%28module%20VHF%29%20MODULE%20ENVIRONMENTAL%20HEALTH?unique=72f3843)
![[KMEDMHDS16-] (mod delivery & neonate) LINEN, single use](/web/image/product.template/572753/image_256/%5BKMEDMHDS16-%5D%20%28mod%20delivery%20%26%20neonate%29%20LINEN%2C%20single%20use?unique=93213d5)
![[KMEDMHHS26-] (mod hospital) LINEN, single use 2015](/web/image/product.template/572825/image_256/%5BKMEDMHHS26-%5D%20%28mod%20hospital%29%20LINEN%2C%20single%20use%20%202015?unique=1b5f516)
![[KMEDMEBO03-] (module VHF isolation) PROTECTIVE EQUIPMENT](/web/image/product.template/571782/image_256/%5BKMEDMEBO03-%5D%20%28module%20VHF%20isolation%29%20PROTECTIVE%20EQUIPMENT?unique=77cac43)
![[KMEDMEBO11-] (module VHF) TRAINING MODULE](/web/image/product.template/571738/image_256/%5BKMEDMEBO11-%5D%20%28module%20VHF%29%20TRAINING%20MODULE?unique=e000fd4)
![[KMEDMEBO17A] (module VHF) SAMPLING AND SMALL ISOLATION](/web/image/product.template/571758/image_256/%5BKMEDMEBO17A%5D%20%28module%20VHF%29%20SAMPLING%20AND%20SMALL%20ISOLATION?unique=e000fd4)