DISSECTOR, OLIVECRONA, 19.5 cm, 4+5 mm 70-35-03
STD
ESURDISO03-
Valid Article
Account code:
60100
HS Code:
901890
Last Updated on:
29/06/2025, 22:43:57
Former
Code(s):
-X
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
L0315 - General surgery dissectors, reusable
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
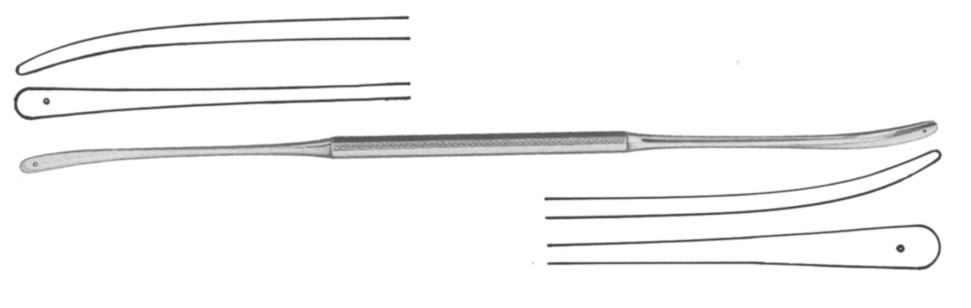
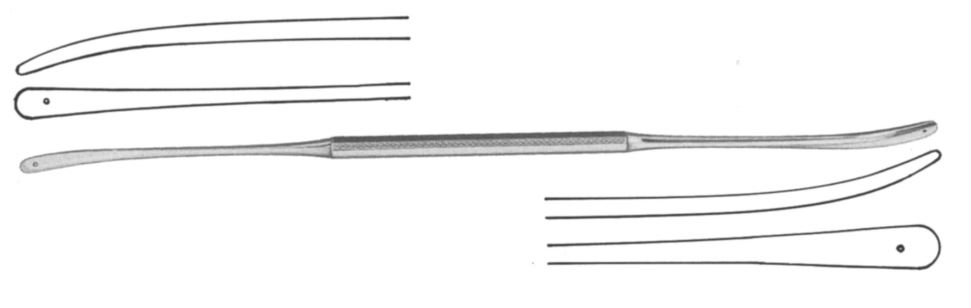
Dissector, Olivecrona
Definition
This dissector is a general-purpose instrument for use in a wide array of neurosurgical procedures. It is double ended making it a highly useful instrument, essentially serving as a two-in-one. It is usually designed with a handle in the middle which continues into a shaft that terminates at the distal end with a rounded curved tip. Each end features a slightly flattened blunt spatula blade that facilitates dissection without causing damage to the tissue area being explored.
Specifications
Technical specifications
- Dissector with eyelet
- Double ended: ends of different width:
- ESURDISO01: 2 and 3 mm
- ESURDISO03: 4 and 5 mm
- Length: 19.5 cm
- Material: martensitic steel
Some restricted information has been hidden. Sign in
to see this information



![[KSURBTRE18-] TREPANATION SET, 18 instruments + Gigli wires](/web/image/product.template/570359/image_256/%5BKSURBTRE18-%5D%20TREPANATION%20SET%2C%2018%20instruments%20%2B%20Gigli%20wires?unique=8b46713)