MANNEQUIN FAMILY PLANNING, IUD insertion
NST
ETMAFAPLIUD
Outdated Article
Account code:
60100
HS Code:
902300
Last Updated on:
21/01/2026, 22:02:40
Former
Code(s):
-X
Y0599 - Learning and training devices - other
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
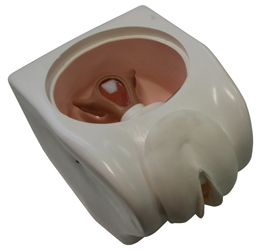
MANNEQUIN FAMILY PLANNING, IUD
Definition
Pelvic block with internal genital organs designed to facilitate demonstration and practice of intra-uterine device insertion.
Specifications
Technical specifications
- Pelvic cavity with anteverted uterus (transparent upper part)
- Cervix with external orifice connected to the uterus
- Abdominal wall made of soft plastic
Packaging & Labelling
Soft carrying bag
Instructions for use
This mannequin allows to practice:
- insertion and removal of intra-uterine device
- evaluation of normal and abnormal uterine positions (anteverted and retroverted)
Storage
Protected from light and dust
MSF requirements
Necessary equipment for training midwives in the field.
Some restricted information has been hidden. Sign in
to see this information
Some restricted information has been hidden. Sign in
to see this information





![[ETMAFAPLIUD2] IUD PLACEMENT MODEL, handheld](/web/image/product.template/582420/image_256/%5BETMAFAPLIUD2%5D%20IUD%20PLACEMENT%20MODEL%2C%20handheld?unique=eb47add)
![[ETMAFAPLIUD3] PELVIC TRAINER, IUD insertion, (3B-P53)](/web/image/product.template/582419/image_256/%5BETMAFAPLIUD3%5D%20PELVIC%20TRAINER%2C%20IUD%20insertion%2C%20%283B-P53%29?unique=eb2abc9)