COMPRESSION BANDAGE, GRADUATED, latexfree, 10 cm x 3.5 m
STD
SDREBANC204
Valid Article
Account code:
60210
HS Code:
300590
Last Updated on:
13/11/2025, 22:11:32
Former
Code(s):
-X SDREBANC051 SDREZBE0051
Single patient multiple use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
M03040101 - Elastic compression bandages, non-adhesive
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
COMPRESSION BANDAGE GRADUATED, light
Definition
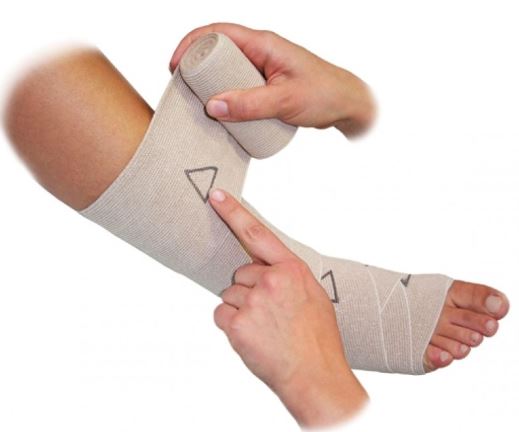
Elastic bandage designed to produce contention or compression used to improve oedema re-absorption and to prevent post-operative thrombosis. Suitable for humerus, tibia, or femoral stumps.
Synonym
graduated elastic bandage, elastic bandage with fitting indicator
Specifications
Some restricted information has been hidden. Sign in
to see this information
Technical specifications
- Two-way elastic bandage
- Woven fabric (latexfree): viscose (59%), elastodiene (23%), polyester (18%)
- Graduated version with tension indicators on the whole length
- Contention class 2 = light (3 layers of thickness)
- Radio transparent
- Non adhesive
- Skin colour
- Non sterile
- Width: 8 or 10 cm
- Length: unstretched 3.5 m, stretched: 4.6 m
Packaging & Labelling
individual packaging in separate box
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Some restricted information has been hidden. Sign in
to see this information
Precautions for Use
This type of bandage can cause severe tissue damage if applied incorrectly.
A compression bandage should not be applied to patients who have marked ischemia or an impaired arterial blood supply.
Do not apply directly on the wound.
Maintenance
- Check the IFU of the manufacturer: washing temperature, drying instructions
- Some bandages may be autoclaved.
MSF requirements
Compression bandage to apply on the stump of an amputated limb in order to reduce post-traumatic oedema.
Some restricted information has been hidden. Sign in
to see this information











![[KMEDMHIS22-] (mod ICU) SPECIFIC DRESSINGS complementary 2021](/web/image/product.template/574343/image_256/%5BKMEDMHIS22-%5D%20%28mod%20ICU%29%20SPECIFIC%20DRESSINGS%20complementary%202021?unique=4195f0f)