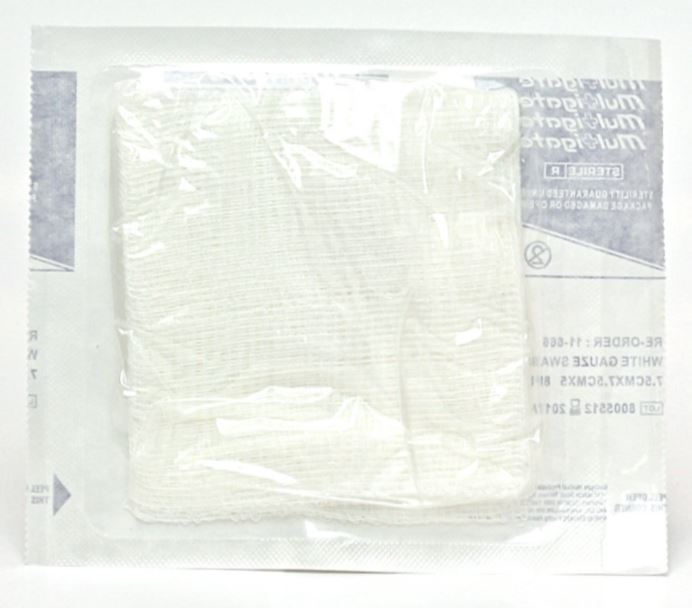
COMPRESS, GAUZE, 10 cm, 12 plies, 17 threads, sterile
Valid Article
GAUZE COMPRESS, woven, sterile
Definition
A non-medicated, sterile dressing item in the form of a patch (also referred to as a sponge) made from woven material and primarily designed to absorb fluids for medical purposes. It is used in wound care, making up of dressings and for any procedure requiring a sterile compress.
Specifications
Quality standards
Technical specifications
- Absorbent gauze, 100 % cotton
- 17 threads/cm²
- Weight: 23 g/m²
- Thickness: 12 plies
- Surgical folding with turned in edges (so that no free thread is apparent after folding or when the first outside fold is opened)
- Sterile, for single use
- Dimensions:
- Large
- Folded: 10 cm x 10 cm
- Unfolded: 30 cm (± 0,5 cm) X 40 cm (± 0,5 cm)
- Small
- Folded: 5 cm x 5 cm
- Unfolded: 20 cm (± 0,5 cm) X 20 cm (± 0,5 cm)
Packaging & Labelling
Packed in a disposable peel pack allowing effective sterilization, safe handling, and storage of all pouched items until needed for use and facilitating proper aseptic presentation of the material.
- per 5 for the 10 cm compresses SDRECOMP1S-
- individually for the 5 cm compresses SDRECOMP5S-
Instructions for use
Compresses intended for sterile medical and surgical care.
It is better to use sterile compresses rather than non sterile compresses that are sterilized in the field. This is important for quality, hygiene, time and cost reasons.










![[KMEDMHHS22-] (mod hospital) DRESSINGS 2015](/web/image/product.template/572827/image_256/%5BKMEDMHHS22-%5D%20%28mod%20hospital%29%20DRESSINGS%20%202015?unique=e7e27ed)
![[KMEDKFAI5RS] MOBILE MEDICAL BAG KIT, rucksack](/web/image/product.template/572106/image_256/%5BKMEDKFAI5RS%5D%20MOBILE%20MEDICAL%20BAG%20KIT%2C%20rucksack?unique=e6a5cca)
![[KMEDMDRS40B] MODULE, DRESSING, 40 dressings, burns](/web/image/product.template/571781/image_256/%5BKMEDMDRS40B%5D%20MODULE%2C%20DRESSING%2C%2040%20dressings%2C%20burns?unique=fc53e25)
![[KMEDMDRS50-] MODULE, DRESSING, 50 dressings](/web/image/product.template/571780/image_256/%5BKMEDMDRS50-%5D%20MODULE%2C%20DRESSING%2C%2050%20dressings?unique=fc53e25)
![[KMEDMEBO08-] (module VHF isolation) MEDICAL SUPPLIES](/web/image/product.template/571731/image_256/%5BKMEDMEBO08-%5D%20%28module%20VHF%20isolation%29%20MEDICAL%20SUPPLIES?unique=ae9ec06)
![[KMEDMLAB118] (laboratory module) MENINGITIS, lumbar puncture, 25 tests](/web/image/product.template/573161/image_256/%5BKMEDMLAB118%5D%20%28laboratory%20module%29%20MENINGITIS%2C%20lumbar%20puncture%2C%2025%20tests?unique=de850e4)
![[KMEDMHOS22-] (mod OT Room) DRESSINGS 2015](/web/image/product.template/572852/image_256/%5BKMEDMHOS22-%5D%20%28mod%20OT%20Room%29%20DRESSINGS%202015?unique=de24307)
![[KMEDMCHO021] (module 001) RENEWABLE SUPPLIES 2019](/web/image/product.template/569222/image_256/%5BKMEDMCHO021%5D%20%28module%20001%29%20RENEWABLE%20SUPPLIES%202019?unique=5bd6309)
![[KMEDMHDS21-] (mod delivery & neonate) RENEWABLE SUPPLIES compl. 2019](/web/image/product.template/569223/image_256/%5BKMEDMHDS21-%5D%20%28mod%20delivery%20%26%20neonate%29%20RENEWABLE%20SUPPLIES%20compl.%202019?unique=5bd6309)
![[KMEDMSUP05S] (IEHK 2024 suppl. module) SUPPLEMENTARY RENEWABLE UNIT](/web/image/product.template/583185/image_256/%5BKMEDMSUP05S%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPLEMENTARY%20RENEWABLE%20UNIT?unique=33019eb)