NEEDLE, s.u., Luer, 19 G (1.1 x 40 mm) cream, IV
STD
SINSNEED19-
Valid Article
Account code:
60210
HS Code:
901839
Last Updated on:
29/06/2025, 23:12:54
Former
Code(s):
-X
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
A0101010102 - Hypodermic syringe needles, w/o safety systems
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
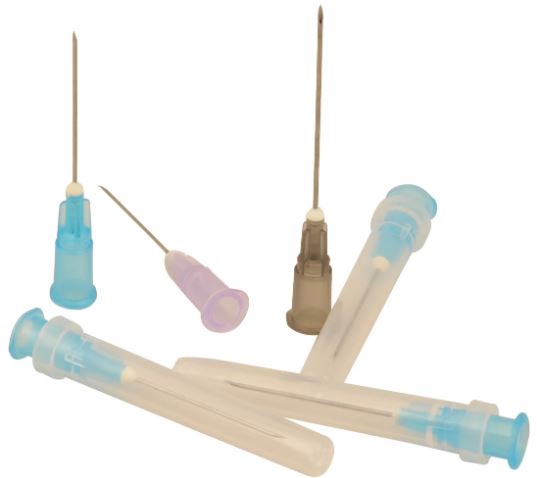
NEEDLE, Luer
Definition
A sterile, sharp bevel-edged, hollow tubular metal device intended to be used in conjunction with syringes or giving sets to prepare and administer fluids/medications/drugs to a patient and/or to withdraw (aspirate) fluids from a patient.
Specifications
Quality standards
- ISO 7864, 2016, edition 4, (confirmed 2021) Sterile hypodermic needles for single use - Requirements and test methods
- ISO 9626, 2016, edition 2, (confirmed 2021) Stainless steel needle tubing for the manufacture of medical devices - Requirements and test methods
- ISO 6009, 2016, edition 4, (confirmed 2021) Hypodermic needles for single use - Colour coding for identification
- ISO 80369-7, 2021, edition 2, Small-bore connectors for liquids and gases in healthcare applications - Part 7: Connectors for intravascular or hypodermic applications
Technical specifications
- NEEDLE
- stainless steel
- tapered bevel
- lubricated
- with protective sheath
- HUB
- polypropylene, etc.
- female Luer connector
- standardized colour code
- Sterile, for single use
Exists in 5 sizes: the Ø is defined by the different ISO standards (including the color code), the length is manufacturer dependent.
| Gauge | nominal outer diameter | length +/- 10% | colour |
| 19 G | 1.1 mm | 40 mm | cream |
| 21 G | 0.8 mm | 40 mm | green |
| 23 G | 0.6 mm | 30 mm | blue |
| 25G | 0.5 mm | 25 mm | orange |
| 26 G | 0.45 mm | 13 mm | brown |
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Precautions for Use
To prevent accidental needlestick injuries, never recap the needle after use.
Some restricted information has been hidden. Sign in
to see this information





![[KMEDKFAI5RS] MOBILE MEDICAL BAG KIT, rucksack](/web/image/product.template/572106/image_256/%5BKMEDKFAI5RS%5D%20MOBILE%20MEDICAL%20BAG%20KIT%2C%20rucksack?unique=919fa2f)
![[KMEDMEBO08-] (module VHF isolation) MEDICAL SUPPLIES](/web/image/product.template/571731/image_256/%5BKMEDMEBO08-%5D%20%28module%20VHF%20isolation%29%20MEDICAL%20SUPPLIES?unique=9ba2a24)
![[KMEDMHCS14-] (mod OPD) INJECTION SUPPLIES](/web/image/product.template/572711/image_256/%5BKMEDMHCS14-%5D%20%28mod%20OPD%29%20INJECTION%20SUPPLIES?unique=93213d5)
![[KMEDMHCS141] (mod OPD) COMPLEMENTARY INJECTION SUPPLIES](/web/image/product.template/572723/image_256/%5BKMEDMHCS141%5D%20%28mod%20OPD%29%20COMPLEMENTARY%20INJECTION%20SUPPLIES?unique=93213d5)
![[KMEDMHHS24-] (mod hospital) INJECTION SUPPLIES](/web/image/product.template/572826/image_256/%5BKMEDMHHS24-%5D%20%28mod%20hospital%29%20INJECTION%20SUPPLIES?unique=4e99d96)
![[KMEDMSUP04M] (supplementary unit 2018) MODULE ANTIMALARIAL ITEMS](/web/image/product.template/572730/image_256/%5BKMEDMSUP04M%5D%20%28supplementary%20unit%202018%29%20MODULE%20ANTIMALARIAL%20ITEMS?unique=c778866)
![[KMEDMLAB1201A] (lab module) TRANSPORT MEDIUM FOR CSF normal 2019](/web/image/product.template/572061/image_256/%5BKMEDMLAB1201A%5D%20%28lab%20module%29%20TRANSPORT%20MEDIUM%20FOR%20CSF%20normal%202019?unique=da8f269)
![[KMEDMNUTO33] (module nut. outpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575294/image_256/%5BKMEDMNUTO33%5D%20%28module%20nut.%20outpatient%29%20MEDICAL%20SUPPLIES%202021?unique=54090d8)
![[KMEDMCHO021] (module 001) RENEWABLE SUPPLIES 2019](/web/image/product.template/569222/image_256/%5BKMEDMCHO021%5D%20%28module%20001%29%20RENEWABLE%20SUPPLIES%202019?unique=c28d635)
![[KMEDMSUP04S1] (supplementary unit 2018) MODULE RENEWABLE SUPPLIES 2019](/web/image/product.template/569226/image_256/%5BKMEDMSUP04S1%5D%20%28supplementary%20unit%202018%29%20MODULE%20RENEWABLE%20SUPPLIES%202019?unique=c28d635)
![[KMEDMHIS24-] (mod ICU) INJECTION SUPPLIES 2021](/web/image/product.template/574424/image_256/%5BKMEDMHIS24-%5D%20%28mod%20ICU%29%20INJECTION%20SUPPLIES%202021?unique=4195f0f)
![[KMEDMHOS33-] (mod OT Room) INJECTION SUPPLIES 2021](/web/image/product.template/574470/image_256/%5BKMEDMHOS33-%5D%20%28mod%20OT%20Room%29%20INJECTION%20SUPPLIES%202021?unique=abfc26b)
![[KMEDMSUP05S] (IEHK 2024 suppl. module) SUPPLEMENTARY RENEWABLE UNIT](/web/image/product.template/583185/image_256/%5BKMEDMSUP05S%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPLEMENTARY%20RENEWABLE%20UNIT?unique=c5e3e98)
![[KMEDMIMM34-] (module immunization 10 000 vacc.) RENEW. MEDICAL SUPPLIES](/web/image/product.template/573263/image_256/%5BKMEDMIMM34-%5D%20%28module%20immunization%2010%20000%20vacc.%29%20RENEW.%20MEDICAL%20SUPPLIES?unique=d6c9d1b)
![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=27e5983)
![[KMEDMSUP05M] (IEHK 2024 suppl. module) SUPPLEMENTARY MALARIA UNIT](/web/image/product.template/583180/image_256/%5BKMEDMSUP05M%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPLEMENTARY%20MALARIA%20UNIT?unique=dacdf9f)
![[KMEDMHMI17-] (mod hospital divers) MEDICINES ANTIMALARIALS inj](/web/image/product.template/572530/image_256/%5BKMEDMHMI17-%5D%20%28mod%20hospital%20divers%29%20MEDICINES%20ANTIMALARIALS%20inj?unique=ae6983d)
![[SINSCONT1R-] REUSABLE SHARPS CONTAINER (RSC), 1.2 litre](/web/image/product.template/569744/image_256/%5BSINSCONT1R-%5D%20REUSABLE%20SHARPS%20CONTAINER%20%28RSC%29%2C%201.2%20litre?unique=248fc0c)
![[SINSCONT4P-] SHARPS CONTAINER, 4 l, plastic, s.u.](/web/image/product.template/569672/image_256/%5BSINSCONT4P-%5D%20SHARPS%20CONTAINER%2C%204%20l%2C%20plastic%2C%20s.u.?unique=ed6ba12)