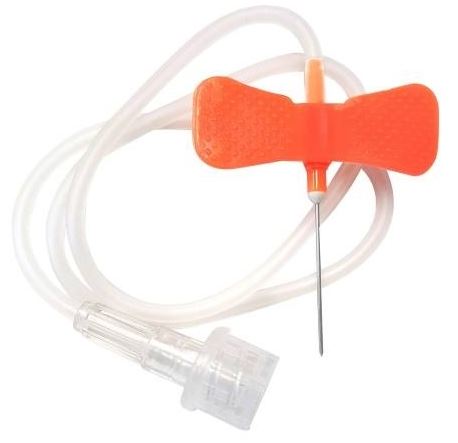
SCALP VEIN INFUSION SET, s.u., 25G (0.5 x 19 mm), orange
STD
SINSSCAV25-
Valid Article
Account code:
60210
HS Code:
901839
Last Updated on:
29/06/2025, 23:13:08
Former
Code(s):
-X
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
A01010202 - Butterfly needles, w/o safety systems
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
SCALP VEIN INFUSION SET
Definition
An invasive device which includes a sharp and rigid needle intended to puncture a vein to collect blood and/or for transient (<24 hours) administration of intravenous fluids. The needle includes wings for fixation/placement and includes a short tubing with a connectors. The device may be referred to as a butterfly needle or scalp vein needle. This is a single-use device.
Synonym
scalp vein needle, butterfly
Specifications
Quality standards
ISO 6009, 2016, edition 4, (confirmed 2021) Hypodermic needles for single use - Colour coding for identification
Technical specifications
- NEEDLE
- stainless steel
- tapered bevel
- lubricated
- length: ± 20 mm
- with protective sheath
- HUB
- polypropylene, etc.
- flexible, folding wings for placing and securing the needle
- standardized colour code
- gauge of the needle is marked on the wings
- TUBE
- transparent, flexible PVC
- length: ± 30 cm
- dead volume: ± 0.5 ml
- ending in a female Luer connector fitted with a stopper allowing an infusion set to be connected
- Sterile, for single use
- Comes in 2 sizes:
- 21 G needle: 0.8 x 19 mm (green), for adults
- 25 G needle: 0.5 x 19 mm (orange), for children
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Please consult the “Manual of nursing Care Procedures, MSF, 2020” available online via the Nursing care working Group sharepoint page.
Precautions for Use
As the needle is sharp, there is a danger of the vascular wall being damaged during infusion.
- The patient must be positioned in such a manner as to ensure immobilization of the puncture site area.
- The puncture site must be regularly controlled during the infusion in order to be able to recognise para-vasal infusions in good time
- For long term infusions, it is preferable to use short catheters which cause less irritation to the veins.
Some restricted information has been hidden. Sign in
to see this information




![[SINSCONT1R-] REUSABLE SHARPS CONTAINER (RSC), 1.2 litre](/web/image/product.template/569744/image_256/%5BSINSCONT1R-%5D%20REUSABLE%20SHARPS%20CONTAINER%20%28RSC%29%2C%201.2%20litre?unique=7d8316b)
![[SINSCONT4P-] SHARPS CONTAINER, 4 l, plastic, s.u.](/web/image/product.template/569672/image_256/%5BSINSCONT4P-%5D%20SHARPS%20CONTAINER%2C%204%20l%2C%20plastic%2C%20s.u.?unique=7d8316b)
![[KMEDMHCS141] (mod OPD) COMPLEMENTARY INJECTION SUPPLIES](/web/image/product.template/572723/image_256/%5BKMEDMHCS141%5D%20%28mod%20OPD%29%20COMPLEMENTARY%20INJECTION%20SUPPLIES?unique=9283e96)
![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=ce93f30)
![[KMEDMHIS24-] (mod ICU) INJECTION SUPPLIES 2021](/web/image/product.template/574424/image_256/%5BKMEDMHIS24-%5D%20%28mod%20ICU%29%20INJECTION%20SUPPLIES%202021?unique=aecada5)
![[KMEDMSUP05S] (IEHK 2024 suppl. module) SUPPLEMENTARY RENEWABLE UNIT](/web/image/product.template/583185/image_256/%5BKMEDMSUP05S%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPLEMENTARY%20RENEWABLE%20UNIT?unique=fa0ffa3)
![[KMEDMHHS24-] (mod hospital) INJECTION SUPPLIES](/web/image/product.template/572826/image_256/%5BKMEDMHHS24-%5D%20%28mod%20hospital%29%20INJECTION%20SUPPLIES?unique=8bbf4fc)