CONDOM, lubricated + RESERVOIR, s.u.
STD
SMSUCOND1--
Valid Article
Account code:
60210
HS Code:
401410
Last Updated on:
07/12/2025, 22:02:03
Former
Code(s):
-X SMSUZFR0053
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
Classification of the medicines in groups and subgroups according to their therapeutic use.
The classification used by MSF is based on the WHO Model List of Essential Medicines.
U110101 - Condoms
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
MALE CONDOM
Definition
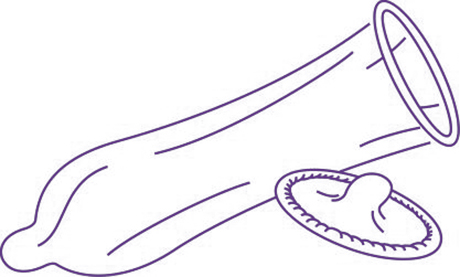
Cylindrical, soft, thin, elastic sheath ending in a reservoir, which fits over the penis.
Used for protection against sexually transmitted infections and for contraception.
Synonym
Durex, French letter, rubber Johnny, sheath, Wellington boot, rubber, raincoat...
Specifications
WHO/UNFPA Technical Specifications for male latex condoms (July 2020)
Quality standards
ISO 4074, 2015, edition 3, Natural latex rubber condoms - Requirements and test methods
Technical specifications
- Natural latex
- Lubricant made of silicone, PEG/N9 etc. with or without starch powder (the manufacturer must specify the level of lubrication)
- With reservoir
- Integral rolled rim
- Smooth texture
- Odourless
- Length: > 160 mm
- Width: 49 - 53 mm, +/- 2 mm
- Rupture resistant:
- Burst pressure > 1 kPa
- Burst volume > 18 dm³ (with a condom > 50 mm and < 56 mm)
- Test made before and after oven-treatment
- Tested for shelf-life and stability
- Non sterile, for single use
Packaging & Labelling
- Unit packaging, opaque, strong, easy to open without damaging the condom.
- Individual package size: 50.8 x 50.8 mm to 57.15 x 57.15 mm
- Inner boxes contain a moisture resistant barrier
- The exterior shipping carton is made from weather resistant corrugated fibreboard
- Minimum labelling requirements: manufacturer's name, batch number (for tracing) and expiry date.
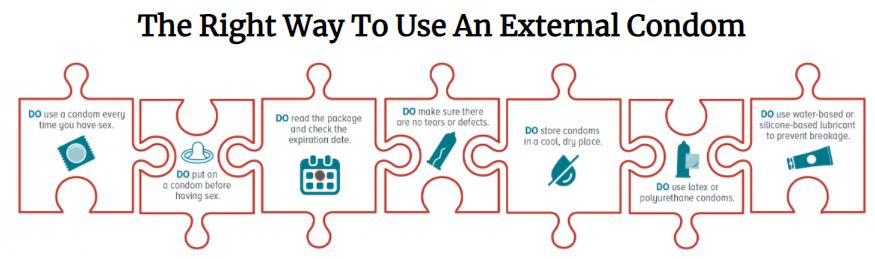
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Precautions for Use
- Never use a condom if the individual package is damaged or defective
- Never use petroleum- or oil-based lubricants (Vaseline, cooking oil, fat, lotion...) since these may damage the latex
- Use only water-based lubricants
- A lubricant (water-based) should always be used for anal penetration
Storage
Store below 30°C, Protect from sunlight ‐ Protect from humidity
Avoid glove compartment, trouser pocket or container left in direct sunlight!
Some restricted information has been hidden. Sign in
to see this information









![[KMEDMTHIS23] (kit TB & HIV start) MEDICINES for opport. infect. 2021](/web/image/product.template/575128/image_256/%5BKMEDMTHIS23%5D%20%28kit%20TB%20%26%20HIV%20start%29%20MEDICINES%20for%20opport.%20infect.%202021?unique=696984e)
![[KMEDMHCS11-] (mod OPD) SMALL RENEWABLE SUPPLIES](/web/image/product.template/572699/image_256/%5BKMEDMHCS11-%5D%20%28mod%20OPD%29%20SMALL%20RENEWABLE%20SUPPLIES?unique=c689d75)
![[KADMKADM2OF] KIT, ADMINISTRATION, office](/web/image/product.template/554230/image_256/%5BKADMKADM2OF%5D%20KIT%2C%20ADMINISTRATION%2C%20office?unique=1dd8980)
![[KADMMLIFB08] (module team life, 8 pers.) BEDDING EQUIPMENT](/web/image/product.template/554191/image_256/%5BKADMMLIFB08%5D%20%28module%20team%20life%2C%208%20pers.%29%20BEDDING%20EQUIPMENT?unique=0b64e01)
![[KMEDMEBO08-] (module VHF isolation) MEDICAL SUPPLIES](/web/image/product.template/571731/image_256/%5BKMEDMEBO08-%5D%20%28module%20VHF%20isolation%29%20MEDICAL%20SUPPLIES?unique=3676d20)