ABSORBENT DRESSING, medium, sterile, s.u.
Valid Article
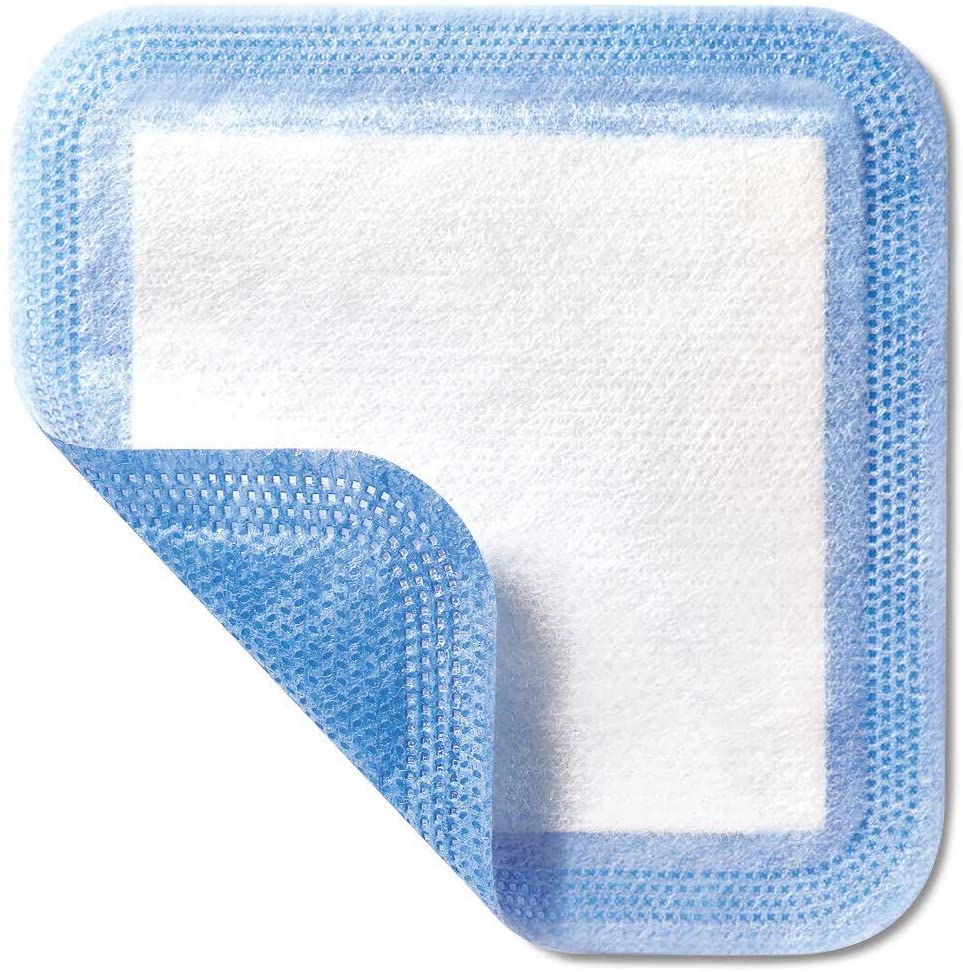
ABSORBENT DRESSING
Definition
A sterile wound covering intended to assist in wound healing by absorbing exudate in wounds (e.g., ulcers, burns, surgical wounds, lacerations, abrasions) and often by creating a moist wound-healing environment. It is not in the form of a gel and does not produce a water-absorbent gel upon contact with wound exudate; it does not contain an antimicrobial agent.
Specifications
Dressing consists of 3 layers
- Non-woven or non-adherent layer: in contact with the wound (reduced risk of adhesion to wound).
- Pad with absorbent fibres: cotton wool, cellulose or viscose, with an integrated distribution layer for even distribution of wound exudate
- Hydrophobic backing: to prevent strike through, in a distinguished colour or marking to avoid confusions with the wound-contact layer.
Quality standards
- EN 13726-1, 2002, Test methods for primary wound dressings - Part 1: Aspects of absorbency
- EN 1644-1, 1997, Test methods for nonwoven compresses for medical use - Part 1: Nonwovens used in the manufacture of compresses
- EN 1644-2, 2000, Test methods for nonwoven compresses for medical use Part 2: Finished compresses
Technical specifications
- hypoallergenic
- breathable
- passive dressing: does not contain any active substances
- free swell absorptive capacity of the dressing: 50 g liquid/100 cm² or 10-15 g liquid/g dressing
- sterile, for single use
- dimensions:
- small: 10 x 10 cm, 15 x 15 cm
- medium: 10 x 20 cm, 15 x 20 cm, 15 x 25cm
- large: 20 x 20 cm, 20 x 25 cm
- exact dimensions vary according to manufacturer
Packaging & Labelling
Each single compress is packed in a disposable peel pack allowing effective sterilization, safe handling, and storage of all pouched items until needed for use and facilitating proper aseptic presentation of the material.
Instructions for use
Precautions for Use
- Fixation is necessary.
- Cannot be cut.
- Should not be used during surgical interventions: use abdominal compresses instead.
MSF requirements
Absorbent sterile dressing that is able to absorb the exudate in moderately to highly exuding wounds.




![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=ce93f30)
![[KMEDMNUTO33] (module nut. outpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575294/image_256/%5BKMEDMNUTO33%5D%20%28module%20nut.%20outpatient%29%20MEDICAL%20SUPPLIES%202021?unique=5c1c774)
![[KMEDMHES35-] (mod emergency) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574355/image_256/%5BKMEDMHES35-%5D%20%28mod%20emergency%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=4c1ecd0)
![[KMEDMHWS31-] (mod ward) MEDICAL RENEWABLE SUPPLIES 2021](/web/image/product.template/574357/image_256/%5BKMEDMHWS31-%5D%20%28mod%20ward%29%20MEDICAL%20RENEWABLE%20SUPPLIES%202021?unique=041b9a6)