ANKLE IMMOBILIZER, double shell
STD
EPHYANIM1--
Valid Article
Account code:
60210
HS Code:
901890
Last Updated on:
29/06/2025, 23:44:53
Former
Code(s):
EPHYZFR0068
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
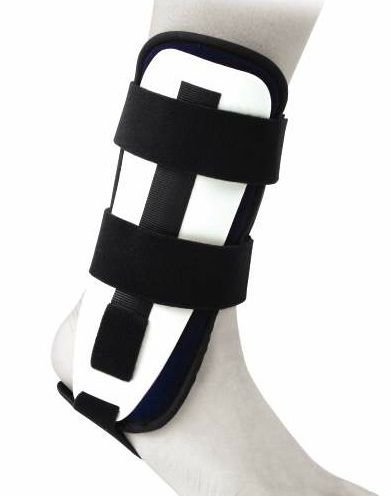
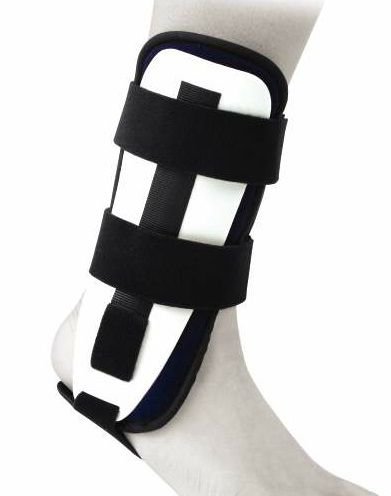
ANKLE IMMOBILIZER, double shell
Definition
A rigid device, used to temporarily render the ankle immovable to support the healing of an injury or surgical wound. This is a bilateral and reusable device.
Synonym
ankle splint, ankle brace
Specifications
Technical specifications
Standard rigid ankle orthosis with a double shell equipped with memory foam protectors for lateral immobilization of the ankle.
- Double shell
- Shape memory foam protectors
- Bilateral positioning possible
- Height adjustable heel strap
- Can have different sizes according to suppliers
- Closure by velcro straps or with hook-and-loop fasteners in malleolar lateral shells.
Packaging & Labelling
Unit package
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Maintenance
Follow the manufacturer's indications for cleaning.
Some restricted information has been hidden. Sign in
to see this information