SPACER, single patient, medium mask
Valid Article
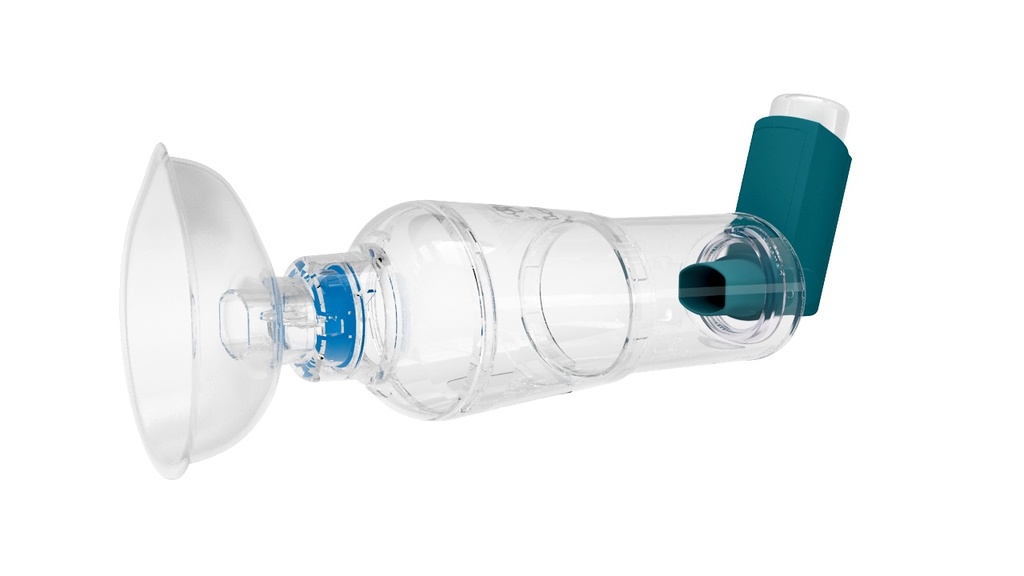
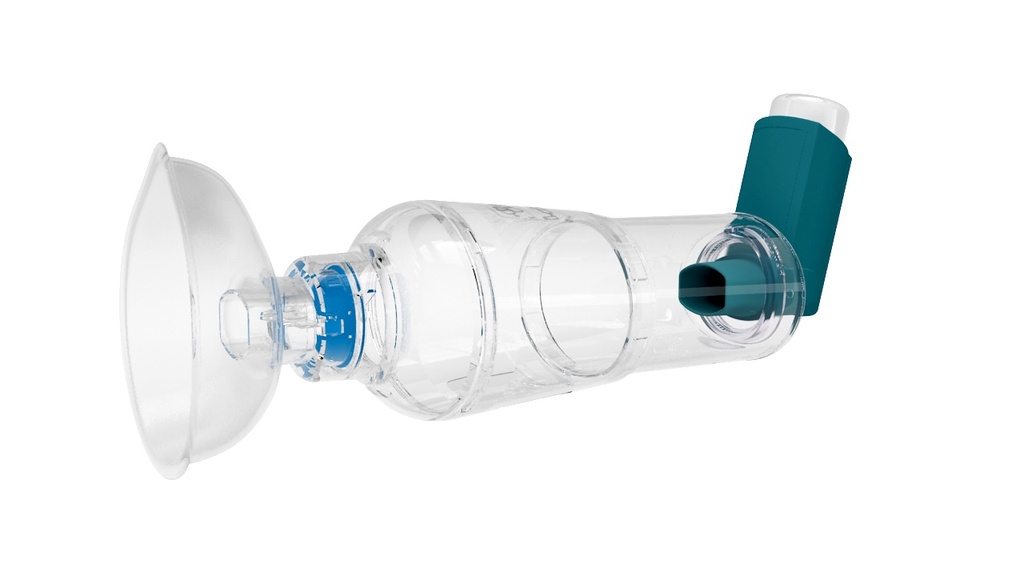
SPACER, single patient
Definition
Medicine chamber spacer for single-patient use
A device intended to be placed between a nebulizer or a metered dose inhaler (MDI) and the patient's mouth, to function as a reservoir into which an aerosol medication is dispensed in order to minimize delivery of large aerosolized particles. By holding the drug after aerosolization this device reduces the direct delivery of large aerosolized particles which would otherwise deposit in the mouth/upper airway, but are intended for the lower airway.
Specifications
Technical specifications
- inhalation chamber washable
- mouthpiece with valve
- facemask in silicone: small or medium
- for single patient use
Packaging & Labelling
Single piece, with 1 face mask included.
Instructions for use
Can be used with or without mask
After spraying medication into the chamber the patient may take the dose either by taking a single deep inhalation or by taking multiple shallow inhalations (tidal breathing). The medication will remain as a cloud for about 10 seconds, so inhalation should follow spraying fairly promptly.
With normal daily use the spacer will last about 12 months, after which it should be replaced.
Precautions for Use
- Clean the spacer and the mask at least once a week according to the manufactures instructions.
- Store the spacer in a clean dry place.
MSF requirements
Cheap spacer, that can be given to the patient when discharged from the hospital.
For a hospital use, where the spacer can be used for several patients, there is a reusable spacer available (see related articles).



![[KMEDMEBO02-] (module VHF isolation) MEDICAL EQUIPMENT](/web/image/product.template/571783/image_256/%5BKMEDMEBO02-%5D%20%28module%20VHF%20isolation%29%20MEDICAL%20EQUIPMENT?unique=d89120d)