BLOOD/FLUID WARMER (Biegler BW-685S), 230V 50/60Hz
Outdated Article
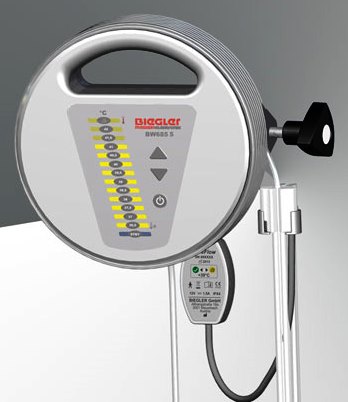
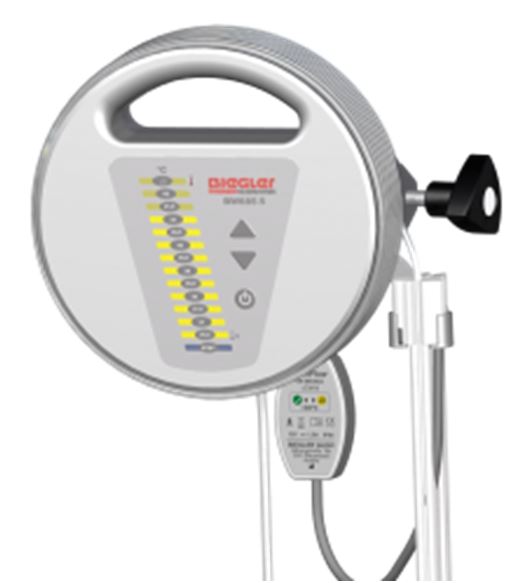
BLOOD/FLUID WARMER (Biegler BW-685S)
The article is replaced by the model 3M RANGER 245 EEMDBFWE4-- (see "replaced by" at bottom of the page).
The BLOOD/FLUID WARMER (Biegler BW-685S) is inadequate for rapid and massive transfusions in trauma and maternal haemorrhage.Hypothermia constitutes one of lethal triad in trauma management and massive transfusion.
The Ranger Fluid Warming unit is capable of administering warmed IV fluid and blood at a very rapid rate (30,000 mL/hr). Current mortality analysis indicates that normothermia is not adequately maintained in these critical situations with standard catalogue items.
Definition
A mains electricity (AC-powered) device designed for heating of blood products or intravenous (IV) solutions, typically from about 4ºC to near body temperature (i.e. 35 to 40 ºC) before infusion, through the conduction of heat.
The device includes a surface heat exchanger for the conduction of heat to the infusion blood/fluid via a disposable tubing set.
The warmer is used to minimize adverse thermal reactions in the patient.
It is mounted with a clamp on an infusion stand under the infusion bag or on a normed rail. It does not infuse blood/fluid.
Specifications
Components
1x BW 685 S (réf: LB22B4685)
1x extension set, box of 100 (réf.: FP4600001)
Technical specifications
- Voltage: 220V-240V AC or 115V AC, 50/60 Hz
- Power consumption: 280 W
- Current: 1.5 A
- Simultaneous warming of multiple transfusions/infusions
- High temperature alarm and low temperature information signal
- Permanent running self-tests
- External service interface for PC
- Mounting on a normed railing and an infusion stand
- Integrated handle
- Type of protection against electric shock: I
- Degree of protection against electric shock: B
- Degree of protection against ingress of liquids: IPX4 splash proof
- Temperature setting: 37° to 41°C in 0.5° C increments
- Over temperature switch-off: 42°C / 42.5°C / 45°C ±3°C
- Audio alarm frequency: 3400 Hz; sound pressure level: 70 dBA
- Low temperature information signal: 250mS / 15 sec
- Max. system pressure: 300 mm Hg
- Safety features: multiple independent cut-off from 42° C
- Fuses: primary 2 x 1.6 AT secondary 500 mAT
- Warm up time: approx. 45 sec.
Dimensions
- W x H x D: 228 x 278 x 132 mm
- Weight: 2.0 kg
To be Ordered Separately
- Extension Set 46000, 460 cm
- Extension Set 35000, 350 cm
- Heating sleeve, Tubeflow LG400TF01. (TubeFlow is an actively heated, reusable silicone profile with control unit for temperature optimization between warmer and patient at low flow rates)
Instructions for use
Read carefully the user manual
Precautions for Use
The length of the tube between the device and the patient must be at least 40 cm, and the tube must not be stretched.
The ambient operating temperature must be in the range of 10° – 30 °C.
Maintenance
Cleaning and disinfection:
- Use a soft cloth with a water-soluble, non-aggressive cleaning agent or a special cleaning agent for plastics
- For disinfection, only use a 0.25 % detergent-disinfectant solution for sufaces (i.e. 20 ml = 1 sachet = 1 stroke of dosing pump for 8 litres water)
- Do not disinfect the device with steam (i.e. in autoclaves), hot air or thermochemical cleaning solutions
Periodic inspections:
Must be carried out at least every 12 months
- checking the warm-up period
- checking the control temperature
- checking the low temperature information signal
- checking the high temperature alarm
- visual check of general condition
- electrical safety
- visual check of general condition (TubeFlow)
- checking the control temperature (TubeFlow)
- Over temperature control (TubeFlow)
Refer to the user manual for more details on how to carry out the checks mentioned above.
MSF requirements
High-performance, microprocessor-controlled device for warming infusions and transfusions to prevent intra- and postoperative hypothermia.

![[EEMDBFWA101] (warmer Biegler BW685S) HEATING SLEEVE Tubeflow LG400TF01](/web/image/product.template/570179/image_256/%5BEEMDBFWA101%5D%20%28warmer%20Biegler%20BW685S%29%20HEATING%20SLEEVE%20Tubeflow%20LG400TF01?unique=6bba655)
![[EEMDBFWC102] (warmer Biegler) EXTENSION SET 460cm, FP4600001](/web/image/product.template/570177/image_256/%5BEEMDBFWC102%5D%20%28warmer%20Biegler%29%20EXTENSION%20SET%20460cm%2C%20FP4600001?unique=eb5a6c3)
![[EEMDBFWC103] (warmer Biegler) EXTENSION SET 350cm, FP1002001](/web/image/product.template/570912/image_256/%5BEEMDBFWC103%5D%20%28warmer%20Biegler%29%20EXTENSION%20SET%20350cm%2C%20FP1002001?unique=1cf8c19)
![[EEMDBFWE4--] BLOOD/FLUID WARMER (3M RANGER 245), 230V 50/60Hz](/web/image/product.template/571526/image_256/%5BEEMDBFWE4--%5D%20BLOOD-FLUID%20WARMER%20%283M%20RANGER%20245%29%2C%20230V%2050-60Hz?unique=5ce6dec)